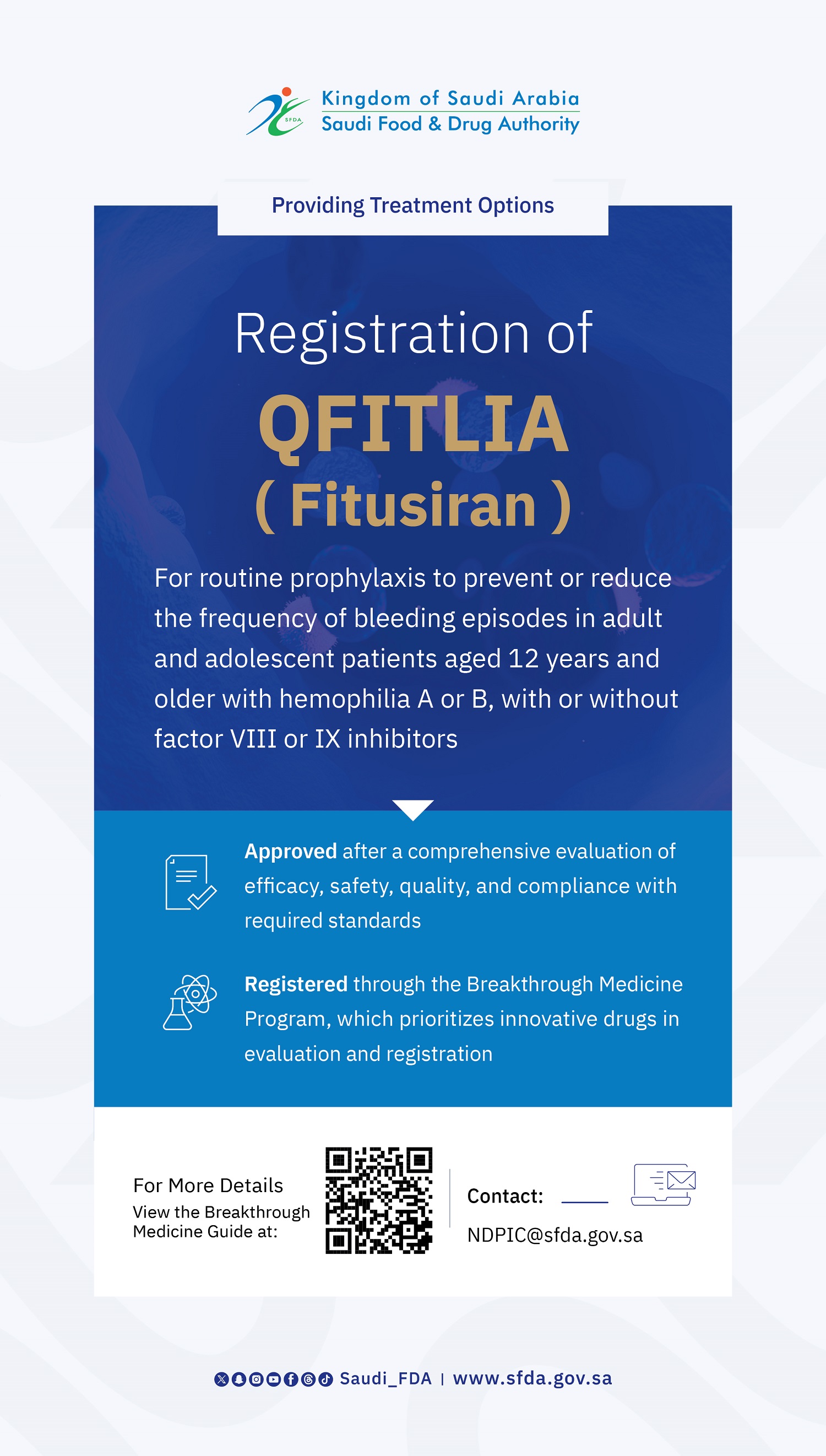
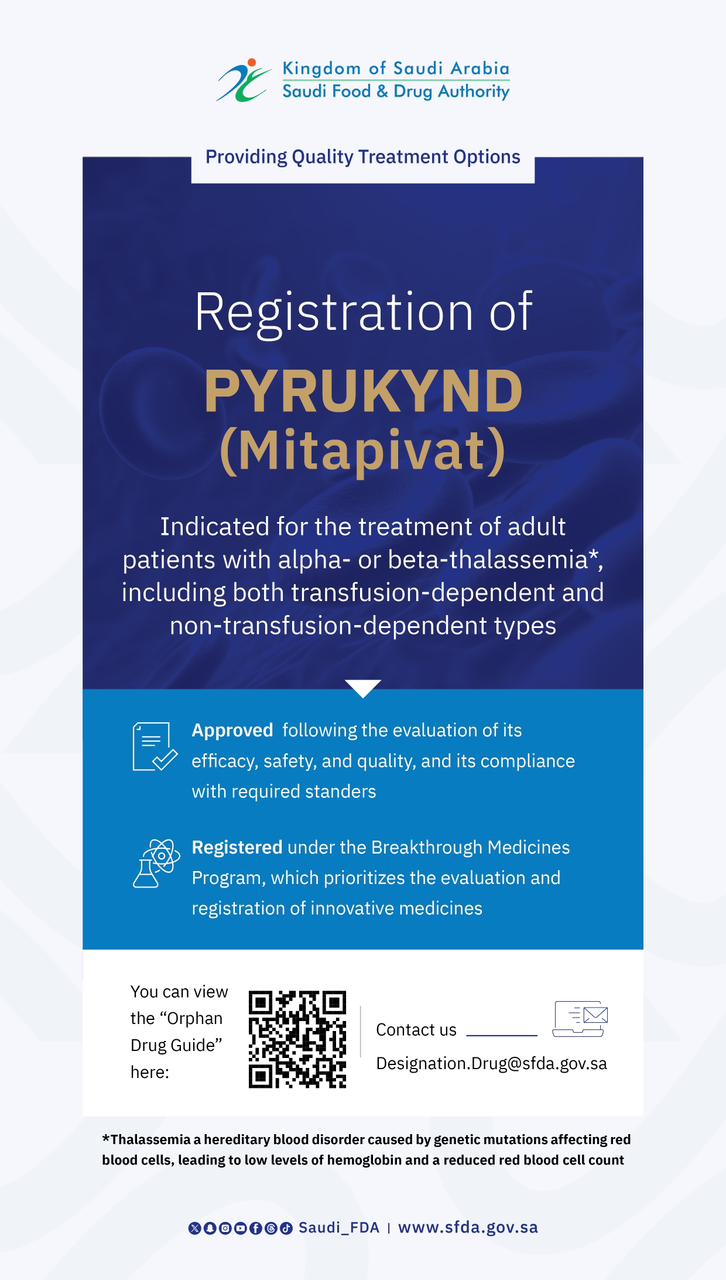
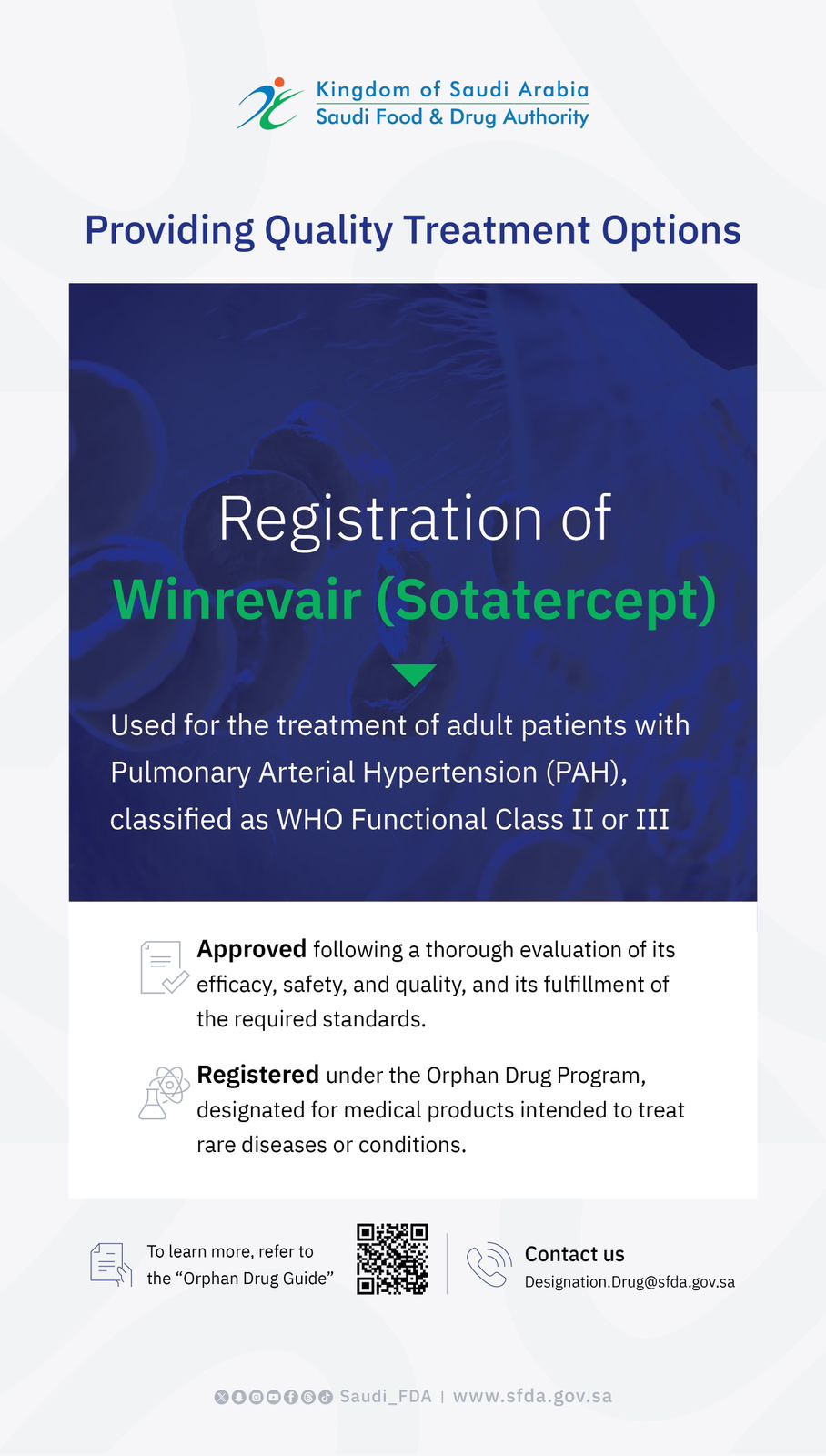
SFDA Grants Orphan Drug Designation to “Sephience”
2026-01-28
The Saudi Food and Drug Authority (SFDA) has granted PTC Therapeutics orphan drug designation to Sephience (sepiapterin), which is indicated for the treatment of hyperphenylalaninaemia (HPA) in patients with phenylketonuria (PKU).
Registration is Subjected to Full Scientific Evaluation
The SFDA clarified that designation under the Orphan Drug Program does not constitute formal product registration. Instead, this designation permits the company to submit a marketing authorization dossier through a dedicated regulatory pathway intended for medicines targeting serious or rare diseases with promising preliminary results. The final registration decision will be announced at a later stage, following the completion of a comprehensive technical evaluation in accordance with applicable regulations.
SFDA Orphan Drug Program Accelerates Access to Critical Therapies
This approval highlights the SFDA's commitment to enhancing access to treatments for rare and chronic diseases through the Orphan Drug Program. This initiative aims to accelerate the availability of breakthrough therapies and address unmet medical needs, thereby improving healthcare quality in alignment with the Health Sector Transformation Program under Saudi Vision 2030.
For further information about the Guidance for Orphan Drug Designation, please visit: (https://www.sfda.gov.sa/en/regulations/88482)