-
About SFDA
About SFDA
SFDA in vision 2030
Authority Strategy
Career and Life
- Information Lists
-
Areas
- Consumer Corner
- Media Centre
- Eservices
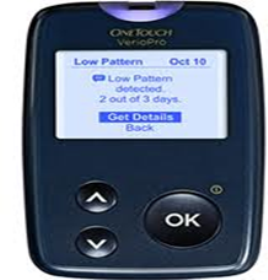
Saudi Food and Drug Authority warns from using OneTouch® Verio® Pro blood glucose meter (home-use)
Saudi Food and Drug Authority warns from using OneTouch® Verio® Pro blood glucose meter (home-use)
Saudi Food and Drug Authority warns from using OneTouch® Verio® Pro blood glucose meter (home-use)
2013-05-23
Saudi Food and Drug Authority represented by the medical devices sector would like to warn all consumers from using OneTouch® Verio® Pro blood glucose meter (home use), manufactured by Lifescan Johnson & Johnson company
SFDA has become aware through Lifescan Johnson & Johnson that they are recalling and replacing all OneTouch Verio Pro blood glucose meter because:
1- The meter does not show a warning at extremely high blood glucose levels of 1024 mg/dL (56.88 mmol/l) and above.
2- The meter display an inaccurate low result at extremely high blood glucose levels of 1024 mg/dL (56.88 mmol/l) and above .
3- There may be a delay in the diagnosis and treatment of severe hyperglycemia or incorrect treatment may be given.
SFDA assures that OneTouch® Verio® Pro blood glucose meter (Home use) did not obtain a marketing authorization from SFDA and haven’t been cleared by SFDA via Saudi Arabia ports of entry.
SFDA has become aware through Lifescan Johnson & Johnson that they are recalling and replacing all OneTouch Verio Pro blood glucose meter because:
1- The meter does not show a warning at extremely high blood glucose levels of 1024 mg/dL (56.88 mmol/l) and above.
2- The meter display an inaccurate low result at extremely high blood glucose levels of 1024 mg/dL (56.88 mmol/l) and above .
3- There may be a delay in the diagnosis and treatment of severe hyperglycemia or incorrect treatment may be given.
SFDA assures that OneTouch® Verio® Pro blood glucose meter (Home use) did not obtain a marketing authorization from SFDA and haven’t been cleared by SFDA via Saudi Arabia ports of entry.
Saudi Food and Drug Authority recommends all consumers and to adhere to the following:
- Discontinue the use of the OneTouch® Verio® Pro blood glucose meters if obtained by any other mean, and use another meter for testing your blood glucose.
- Call Lifescan customer services at 8002440266 to receive a replacement meter at no charge if obtained by any other means.
- Inform the Saudi Food and Drug Authority if you have affected product through NCMDR website:
- Discontinue the use of the OneTouch® Verio® Pro blood glucose meters if obtained by any other mean, and use another meter for testing your blood glucose.
- Call Lifescan customer services at 8002440266 to receive a replacement meter at no charge if obtained by any other means.
- Inform the Saudi Food and Drug Authority if you have affected product through NCMDR website:
Saudi Food and Drug Authority encourages all to report any adverse event associated with medical devices through the National Center for Medical Devices Reporting :
For more information