SFDA Issues Warning Against Specific Nestlé Infant Formula Products
2026-01-06
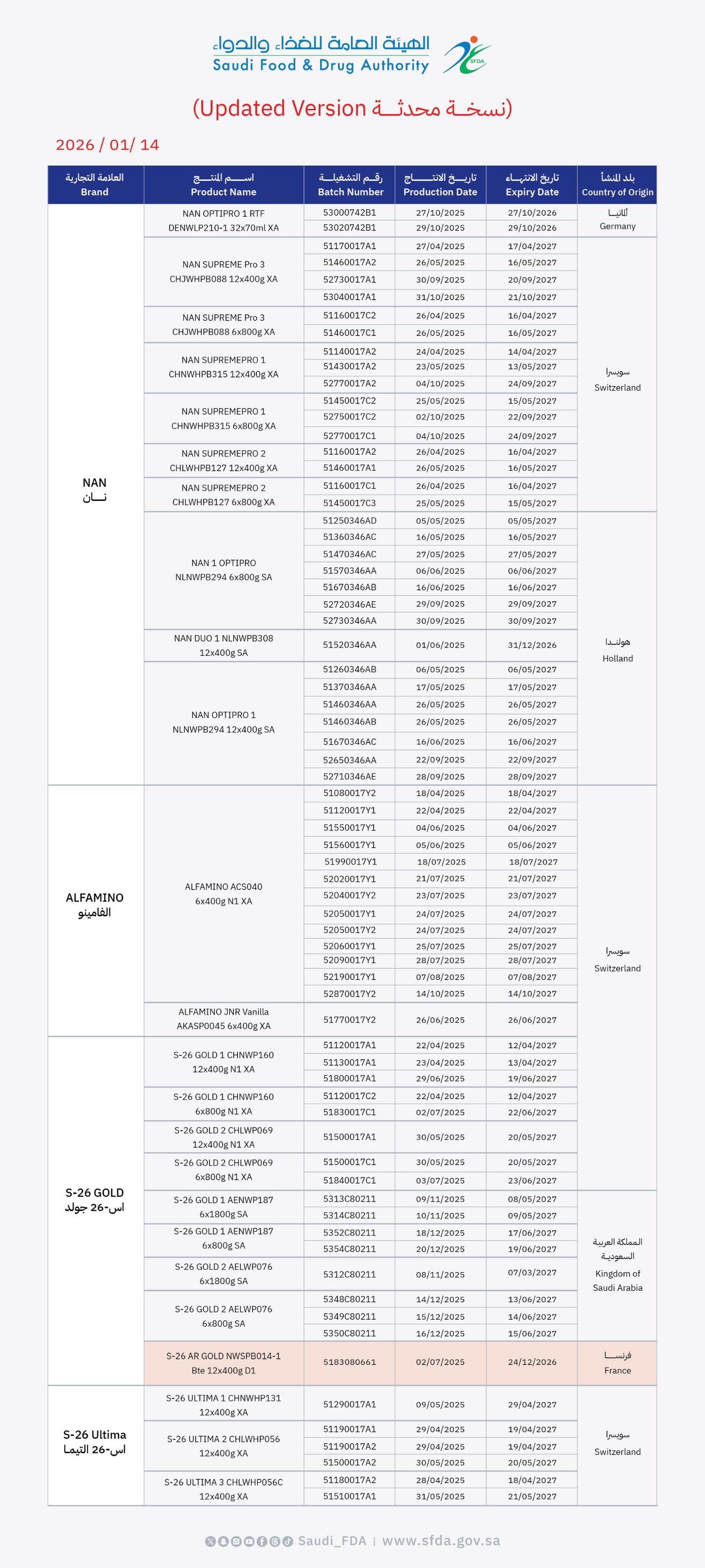
The Saudi Food and Drug Authority (SFDA) has issued a warning regarding specific infant formula products produced by Nestlé under the NAN, ALFAMINO, S-26 GOLD, and S-26 ULTIMA brands. This warning follows a voluntary notification from the company concerning a recall of several product batches due to potential contamination with cereulide, a toxin produced by Bacillus cereus bacteria, which may pose a risk to infants’ health.
The SFDA clarified that this voluntary recall is a precautionary measure implemented after an assessment of potential health risks. Exposure to cereulide toxins can lead to symptoms such as nausea, frequent vomiting, and abdominal pain. However, the Authority confirmed that no reports of illnesses associated with these products have been received in Saudi Arabia to date.
The SFDA advises consumers to stop using the recalled batches and dispose of the products immediately. The Authority stated it has taken the necessary measures and is currently overseeing the withdrawal of these products from the market in direct coordination with the company. The attached table provides the product details and batch numbers included in the recall.
The SFDA affirmed its commitment to monitoring food safety and ensuring strict compliance with health regulations. Citizens and residents are urged to report any food-related violations or concerns through the Unified Call Center (19999) or the "Saudi Vigilance" service.