Post-Market Clinical Evaluation for the Safety of Bovine and Porcine Pericardial Valves
Post-Market Clinical Evaluation for the Safety of Bovine and Porcine Pericardial Valves
Bio-prosthetic mitral valves were introduced in the 1970s to the clinical market for the treatment of valvular heart diseases. Since that time, there was an increasing trend towards the usage of bio-prosthetic valves not only for elderly patients, but also for younger patients to overcome the necessity of lifelong anticoagulation associated with the mechanical valve [1]. There are various parameters to be considered for selecting the most suitable bio-prosthetic mitral valve, which vary from a patient to another, based on the clinical need. However, among the most important feature to be considered is the level of valve degradation, which directly impact the outcomes and survival of patients [2]. This study was conducted to evaluate the safety and durability of porcine and bovine valves, when placed at the mitral position, as revealed by the available published articles and the inputs from the saudi's practitioners at healthcare facilities.
Part I: Clinical need of mitral valve
The human heart consists of four valves, which determine the pathway of blood flow during systolic and diastolic phases of the cardiac cycle. The native mitral valve is located in the left side of the heart, which is considered as a blind spot between the left atrium and left ventricle as it appears in figure 1. In normal diastolic cycle, the mitral valve opens to allow blood to flow from the left atrium to left ventricle, but closes during normal systolic phase to prevent the passing of blood backward with left ventricle contraction [4].
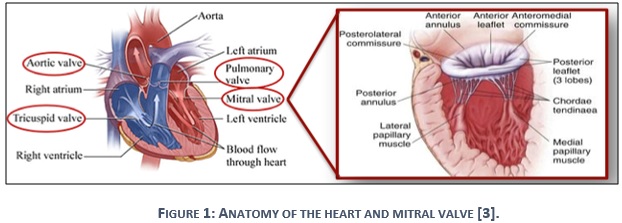
Part II: Indications for mitral valve replacement (MVR)
In some cases, mitral valves do not open or close properly, which disrupt the blood flow through the heart to the body. Abnormal conditions of the mitral valve are characterized as either mitral regurgitation or mitral stenosis [5]. Valve regurgitation occurs when blood leaks backward into the left atrium while the leaflets are closed. The regurgitation happens due to leaflets fail to close tightly, and results in heart failure, atrial fibrillation, or pulmonary hypertension [5]. While mitral stenosis appears when the orifice of the valve is narrowed to pump out blood to the left ventricle due to thickness of the valve tissue, which drives to pulmonary hypertension, heart failure, heart enlargement, atrial fibrillation, or blood clots [6]. The diseased mitral valve can be replaced by an artificial mitral valve, considering the severity of the disease. There are two main types of artificial mitral valve, mechanical valves and bio-prosthetic (tissue) valves.
- Mechanical valves are long lasting valves, which normally last throughout the patients’ lifetime, and require anti-coagulation to avoid clot forming [2].
- Bio-prosthetic valves are made from animal donor valves, which should be strong and flexible. These valves can last for 10-20 years, and normally do not require the long-term use of medication [2].
Part III: Intended use of the artificial bio-prosthetic mitral valve.
Bio-prosthetic mitral valve is intended to replace a malfunctioning native or prosthetic mitral heart valve. It is originally obtained from an animal tissue (xenograft), most commonly porcine or bovine pericardial. The valve is designed to be implemented by surgeons through median sternotomy or minimally invasive heart surgeries [8] [9].
The essential purpose of the methodology of this evaluation is to compare the durability and safety levels of bovine pericardial compared to porcine aortic (and mitral) valves through clinical paper review, and the clinical experience review. The clinical paper review aims to review the currently published academic papers related to the topic, while the clinical experience review explores the opinions of other regulatory offices, and most importantly the opinions of the local societies and experts located in the kingdom of Saudi Arabia.
Part 1: Clinical Paper Review (literature review)
1.1 Search criteria
A search was conducted in 2020 through PubMed and Google scholars. We applied the PICO model to specify the search inclusion criteria. The abstracts of the obtained articles were screened in reference to the inclusion criteria, and then, extra quality measures were considered for the remained articles. Only articles that were written in English, and designed to compare the porcine and bovine valves in term of the safety and durability were considered.
1.2 Results
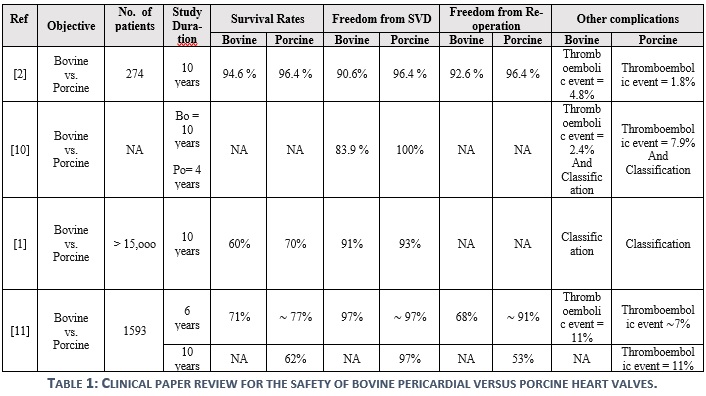
Four articles were acquired to build the findings of this review, as demonstrated in table 1. Results suggest that both bovine and porcine valves are demonstrating similar durability, but there are significant variations in the studies for the associated complications. It was reported by some experts that the durability of porcine valves is higher than that of the bovine valves at the mitral position. Such a claim could not be supported by the reviewed articles, but rather, both products were found to be durable for sufficient time with a minor and not significant variation. However, only 1 out of 4 studies (Ref. 11) shows a noticeable difference with respect to the freedom from reoperation in favor to porcine valves, which does not facilitate a strong evidence.
Part 2: Saudi Users' Experiences
Five Surgeons from Saudi's hospitals were consulted to provide their opinion regarding the safety of bovine pericardial valve compared to porcine aortic (and mitral) valve. While all those experts have enough experience in using both bovine and porcine valves, two of them believe that the porcine valve is demonstrating better durability in the mitral valve position. Some surgeons stated that the complication rates could be the same for all brands, expect in durability level, struts profile, and orientation; however, mortality rates are similar in bovine pericardial and porcine valves, as theses rates depend on the age, ventricular function of the patient, and multiple other factors, but not the origin of the valve material.
Part 3: Conclusion
The overall conclusion suggests that both bovine pericardial and porcine aortic (and mitral) valves are proven to be safe whenever valves are implemented as indicated by the manufactures' recommendations.
This study is authored by Eng. Jumana Masoudi, who designed the survey, collected and analyzed data, and wrote up the manuscript of the study. Eng. Bader Al-oufi monitored the study progress. With the appreciation to the post-market clinical evaluation team for their efforts in conducting this work.
We also acknowledge the contribution of the following experts, who provided valuable and significant inputs to the study context:
- Dr. Zohair Alhalees - King Faisal Specialist Hospital and Research Center/ Riyadh
- Dr. Othman Alothman - King Faisal Specialist Hospital and Research Center/ Jeddah
- Dr. Nasser Alkhamees - King Saud University Hospital/ Riyadh
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] Pietro Giorgio Malvindi, MD, PhD, Florinda Mastro, MD, Mariusz Kowalewski, MD, Margot Ringold, MD, Vito Margari, MD, Piotr Suwalski, MD, PhD, Giuseppe Speziale, MD, PhD, and Domenico Paparella, MD, "Durability of Mitral Valve Bioprostheses: A Meta-Analysis of Long-Term Follow-up Studies," The Society of Thoracic Surgeons, 2020.
[2] Karthik Raman & Anbarasu Mohanraj & Vijayanand Palanisamy & Bharat Kumar Mohandoss & Sivakumar Pandian & Anjith Prakash Rajakumar & Jacob Jamesraj & Ejaz Ahmed Sheriff & Valikapathalil Mathew Kurian & Rajan Sethuratnam & Ravi Agarwal, "Porcine versus bovine bioprosthetic valves in mitral position: does choice really matter?," Indian Journal of Thoracic and Cardiovascular Surgery, vol. 36, no. 2, pp. 105-113, 2020.
[3] CardioSmart, "Your heart has four valves and problems can arise when one or more of the valves aren't opening or closing properly. Read this helpful guide to learn everything you need to know about valve disease.," American Collage of Cardiology, 2020.
[4] Joshua D. Pollock; Amgad N. Makaryus., "Physiology, Cardiac Cycle," StatPearls Publishing, 2019.
[5] Mayo Clinic, "Mitral valve regurgitation," 2020. [Online]. Available: https://www.mayoclinic.org/diseases-conditions/mitral-valve-regurgitation/symptoms-causes/syc-20350178. [Accessed 01 03 2020].
[6] Mayo Clinic, "Mitral valve stenosis," 2020. [Online]. Available: https://www.mayoclinic.org/diseases-conditions/mitral-valve-stenosis/symptoms-causes/syc-20353159. [Accessed 01 03 2020].
[7] Mayo Clinic, "Mitral valve repair and mitral valve replacement," 2020. [Online]. Available: https://www.mayoclinic.org/tests-procedures/mitral-valve-repair-mitral-valve-replacement/about/pac-20384958. [Accessed 01 03 2020].
[8] LivaNova, "Pericarbon MORE Mitral," LivaNova PPT Presentation, 2020.
[9] Hussein Khalidieh, "Mosaic: Undeniable Durable," Medtronic PPT Presentation, 2020.
[10] Jagdish Butanya,b,*, Cristina Fayeta, Manmeet S. Ahluwaliaa, Patrick Blita, Christina Ahna,, "Biological replacement heart valves identification and evaluation," Cardiovascular Pathology, vol. 12, pp. 119-139, 2003.
[11] L. Conrad Pelletier, MD, Michel Carrier, MD, Yves Leclerc, MD, Gilles Lepage, MD, Pierre deGuise, MD, and Ihor Dyrda, MD, "Porcine Versus Pericardial Bioprostheses: A Comparison of Late Results in 1,593 Patients," The Society of Thoracic Surgeons, pp. 352-361, 1989.


