An Evaluation for the Most Common Issues Associated with Anesthesia within Saudi Healthcare Facilities
An Evaluation for the Most Common Issues Associated with Anesthesia within Saudi Healthcare Facilities
Misuse of medical devices can generate series of adverse events that may result in serious incidents. Therefore, SFDA defined certain requirements to enable a safer use of medical devices within healthcare facilities, as clarified in the published guideline: “SFDA Requirements for Quality, Safety, and Effectiveness of Medical Devices at Healthcare Facilities”. These requirements include some practices that relate to the use of medical devices, beside others that aim to reduce the misuse in certain critical devices, such as anesthesia machines. The essential aim of this study is to identify the significant issues of anesthesia machine during its operational lifecycle within 140 healthcare facilities, as revealed by the status of compliance to the evaluation requirements. Moreover, common issues will be identified to be targeted in recommendations to guide related parties toward enhancing the device utilization.
Part I: Methodology
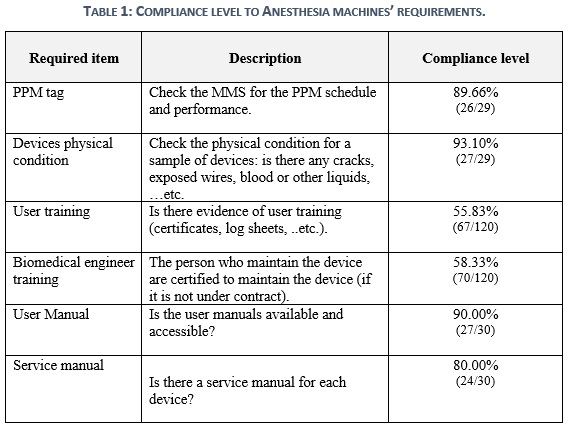
Data of this study are extracted from the evaluation reports of 140 Saudi healthcare facilities. In the first year, 50 healthcare facilities across Saudi Arabia were evaluated through on-site evaluations, whereas during the second year, 90 healthcare facilities were evaluated remotely, considering the consequences of the COVID-19 pandemic. Each site was evaluated considering the safe-use requirements of anesthesia, which include; preventive maintenance tag, devices physical condition, user raining, biomedical engineer training, user manual, service manual. Moreover, the most and least common issues that were resulted due to their significant non-compliance level to anesthesia requirements are analyzed over 30 HCFs, and each site was evaluated using the same 6 parameters.
Part II: Results
Table 1 shows the compliance-level of Saudi healthcare faculties. The results indicate a non-compliance of 10% to the PPM procedures within all sample of healthcare facilities. Also, it was found that there is 7% non-compliance to the devices physical condition within the anesthesia. However, a high level of non-compliance with the safe use of anesthesia results in 44% for the user training and 42% for the biomedical engineer training. Not only that, but the same case is also concluded for the availability of user and service manuals by non-compliance percentages of 10% and 20%.
2. Common technical issues associated with anesthesia machines
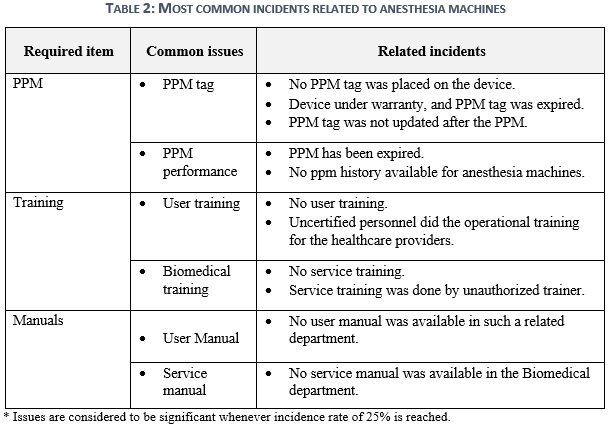
This section aims to sum up the previous statistics to indicate the most and least common issues of SHCF that are in non-compliance with SFDA requirements for critical medical device. Table 2 indicates that the most common issues of anesthesia are related to PPM, Training and manual. PPM activity shall be performed properly in reference to manufacturer recommendations to ensure the ideal life span, well-functioning, and safety of healthcare providers and patients. Upon that, acquiring deficiencies through PPM performance or in ppm tag with a frequency of 27% will impair the healthcare facility in total. Technical and operational pieces of training present a prime opportunity to expand the knowledge base of healthcare providers in operating and utilizing the anesthesia machine. Nonetheless, the anesthesia machine is not like any other tool, it is a life support machine that will perform well when operators know how to use it properly. Consequently, any technical or user training neglect with 40% and 33% occurrence repetition will elevate the risk of adverse events and recalls. The role of service and user manuals with medical devices is to comprehend how the anesthesia works technically and clinically. Not only that, but having also written manuals will ease the route to obtain information, transfer knowledge, and self-learning. Thus, having a shortage of manuals in the healthcare facilities would point out the lack of knowledge in implementing the clinical and technical practices into and from the medical devices, and recurring this issue by 30% for user manual and 40% for service manual will increase it.
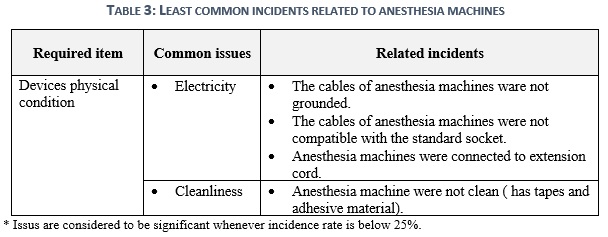
The devices physical condition of the anesthesia machine is the main interface of the healthcare facility to represent the quality provided to patients. It will also mirror the overall image of how the safety of critical medical devices is being performed in their hospitals. Hence, the re-existence by 13% of residue bio-fluids and improper electricity will not only reflect improper quality, but they will also impact negatively on healthcare provider’s life in first followed by patient’s life table 3.
Part III: Conclusion
The analyzed results indicate that healthcare providers shall adopt SFDA requirements to ensure patient safety along with user safety from the risks associated with anesthesia machines. Moreover, healthcare providers must follow the manufacturer recommendations to avoid the misuse of the device that may lead to incidents, and to use the device in the best proper way.
Recommendations for Healthcare Providers
1. Attain training from certified personal:
a. Service trainings for all biomedical engineers and technicians who maintain and fix medical devices according to the manufacturers recommendations, and to restrict handling the devices by those who are NOT certified by the device manufacturers.
b. Application trainings to all end-users who are using anesthesia devices based on the manufacturers recommendations, and to restrict using the devices by those who are NOT certified by the device manufacturers.
2. Obtain the original anesthesia manuals:
a. Service manual to be accessible and used by biomedical engineers and technicians for all models of anesthesia.
b. User manual to be accessible and used by end users for all models of anesthesia.
3. Periodic preventive maintenance (PPM) of anesthesia devices:
a. Perform the PPM according to frequency provided by the manufacturer.
b. Restrict to the commitment of not exceeding the ppm due date.
c. Update PPM tags of all anesthesia devices even those who are under warranty or service contract, which matches the PPM record in the maintenance management system (MMS).
d. Update the MMS to provide PPM-history to track the PPM schedule of anesthesia machines.
4. Follow SFDA recommendations:
a. According to the safe use of medical devices, avoid using adapter, extensions, , and plugs are not compatible with the hospital sockets or not grounded.
b. Routinely check the decontamination procedures after PPM, CM, patient use.
Grateful thanks to Eng. Jumana Masoudi, who designed the study, worked out the data collection and analysis, and wrote up the study context. Eng. Bader Aloufi verified the study methodology and supervised the study progress. With the appreciation to Sara Alharthi for drafting this summary and the post-market clinical evaluation team for their efforts in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] Requirements for Quality, Safety and Effectiveness of Medical Devices at Healthcare Facilities, Saudi Food and Drug Authority, 2019


