SFDA Warns from using Five Hair Straightening Products as they Contain High Percentage of Formaldehyde
2016-11-14
SFDA Warned consumers from using Five Keratin- Hair Straightening Products as they Contain High Percentage of Formaldehyde.
SFDA announced that its specialists had collected and analyzed samples of keratin hair straightening products from the local market to check for their safety, in line with SFDA program regarding control and safety of locally marketed cosmetic products, based on Cosmetics Regulations issued by the Royal Decree Number M/49 dated 18/6/1436H.
. SFDA added that the results of these analysis revealed that five of these products contain high percentage of Formaldehyde which is considered dingoes if exceeded 0.2% ( The permissible concentration by GSO 1943/2009) lading to eye irritation, respiratory system disorder-including cough & breathing difficulty- and may cause cancer on the long run.
The first product is called (Amazon x-treme Brazilian Keratin Treatment), made in USA with Lot number (AXSA032515) and expiry date 03/01/2017
The second product is called (Brazilian Keratin Technology Advanced Formula), made in Brazil with Lot number (7643) and expiry date 04/03/2019
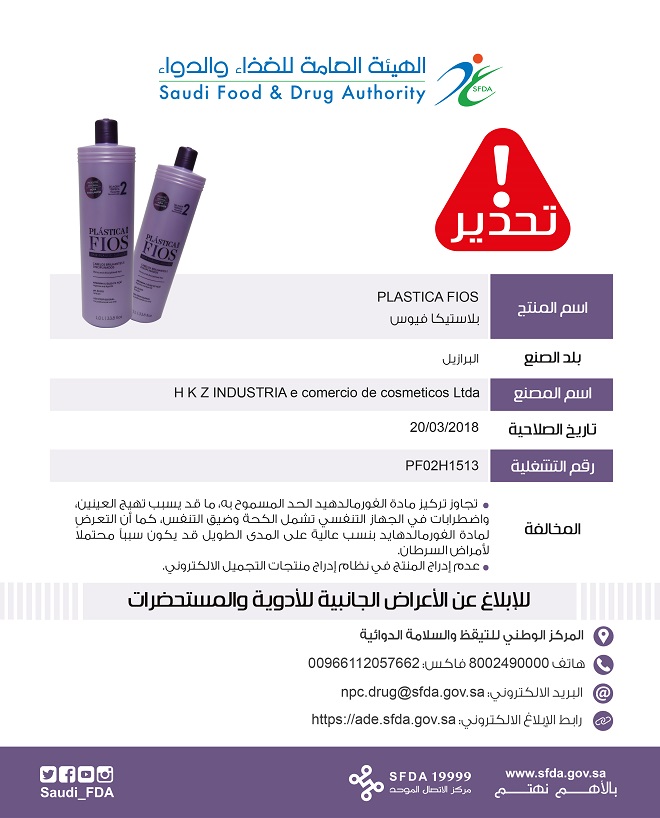
The third product is (PLastica Fios), made in Brazil with Lot number (PF 02H 1513) and expiry date 20/03/2018
The fourth product is (NUTRIMAX ATIVA S DA AMAZONIA), made in Brazil with Lot number (039) and expiry date 30/12/2017
The fifth product is called (AGI MAX KERA-X), made in USA with Lot number (124) and expiry date 01/06/2017
SFDA advised consumers not to use these products – in the lot numbers shown above- and to dispose of any quantities they may have in hand, stressing the importance of buying products from reliable sources through which products origin can be traced.
However, points of sales, distribution centers and ladies beauty saloons should stop selling these products and return them to importers. Moreover, importers and distributors should recall these products from the points of sale and warehouses and shall coordinate with SFDA to destroy them.
SFDA addressed the respective authorities to track recall of these products from the market, prevent their entry and take the necessary disciplinary actions against violators.