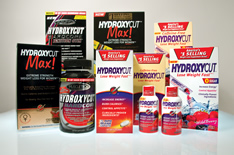
The SFDA Warns against Using (Hydroxycut) Drugs
2009-05-09
The SFDA warns against using (Hydroxycut) products which are manufactured by (Lovate Health Sciences) Company, as they may cause severe side effects on the lever. The company has withdrawn its products from the American markets according to 23 notifications submitted to the FDA since 2002 to 2009. Sever side effects include increase in lever enzymes and icterus which indicate lever disorder and one death case due to lever failure.
The SFDA in the Kingdom draws the attention of consumers to stop using Hydroxycut products in order to prevent any side effects.
These products are used as supplements that contain several ingredients and unknown herbs extracts. They are marketed under medical advertisements as: weight loss, fat burning, energy enhancement, reducing carbohydrate absorption, getting rid of excess water in the body.
It worth mention that these products have the coming trade names:
Hydroxycut Regular Rapid Release Caplets
Hydroxycut Caffeine-Free Rapid Release Caplets
Hydroxycut Hardcore Liquid Caplets
Hydroxycut Max Liquid Caplets
Hydroxycut Regular Drink Packets
Hydroxycut Caffeine-Free Drink Packets
Hydroxycut Hardcore Drink Packets (Ignition Stix)
Hydroxycut Max Drink Packets
Hydroxycut Liquid Shots
Hydroxycut Hardcore RTDs (Ready-to-Drink)
Hydroxycut Max Aqua Shed
Hydroxycut 24
Hydroxycut Carb Control
Hydroxycut Natural
According to the above mentioned; the SFDA warns against using or buying these products or bringing them from abroad as they are not registered in the Kingdom.
Citizens may have more information about the severity of herbs mixtures