SFDA Alerts About Error in ‘First Step’ Pregnancy Test Instructions for Use
2015-09-10
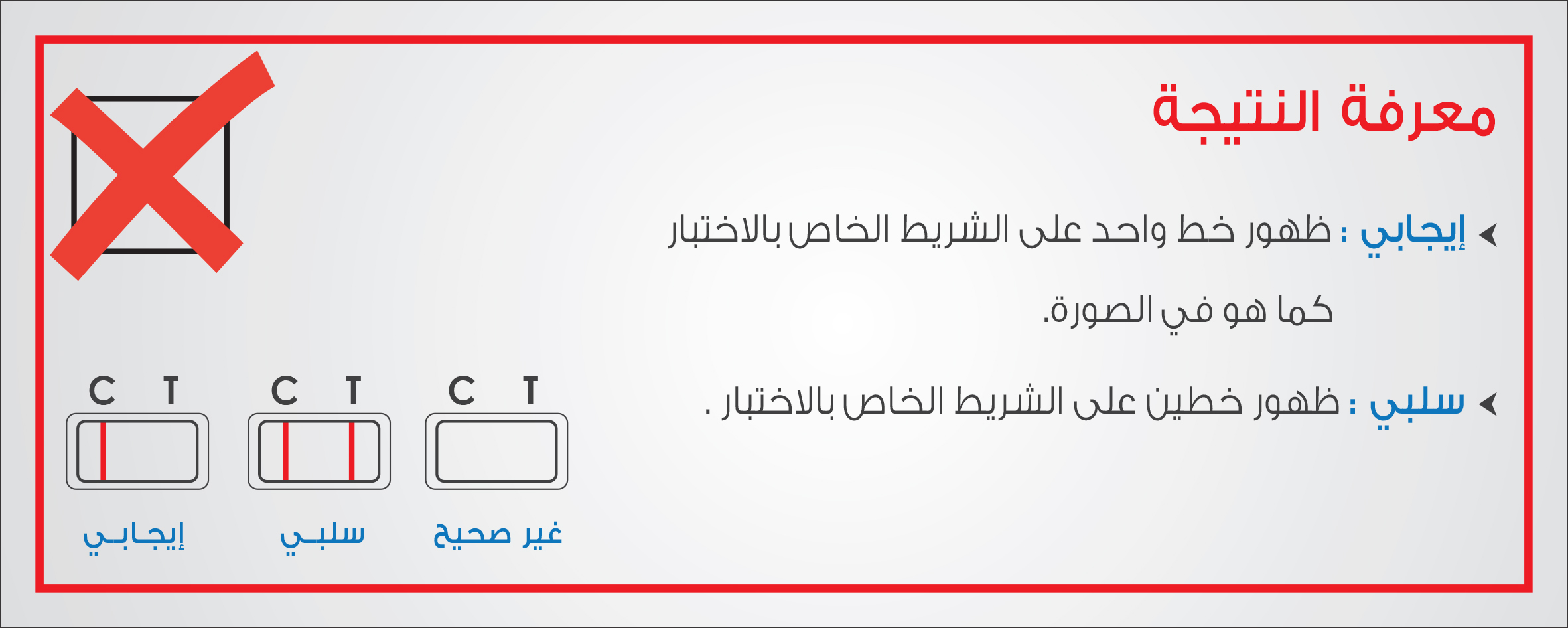
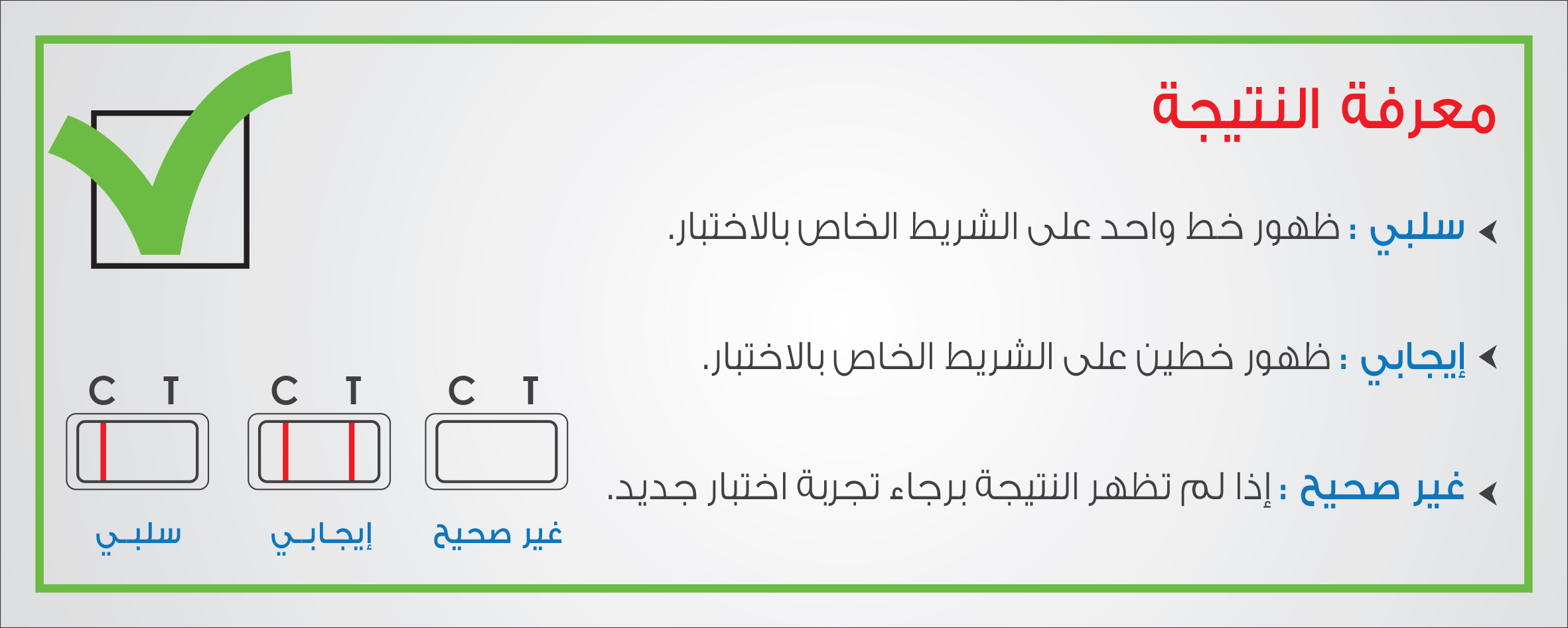
SFDA clarified that the device, manufactured by ‘Aid Diagnostic’, is used for early detection of pregnancy by determining HCG hormone in urine samples, pointing out that a mistake occurred in Lot No. C6-130417, where the Instructions for Use attached to the device indicated that the test result will be Positive (1) for pregnant woman and will be Negative (II) for non-pregnant woman. SFDA noted that the use of the affected product can be continued observing that pregnancy rest result will be Positive (II) for pregnant woman and will be Negative (I) for non-pregnant woman, recommending the importance of referring to a Healthcare facility in case the accuracy of the test result is doubted.
SFDA called on users to report medical devices& products problems by visiting the National Center for Medical Devices Reports Website at: http://ncmdr.sfda.gov.sa