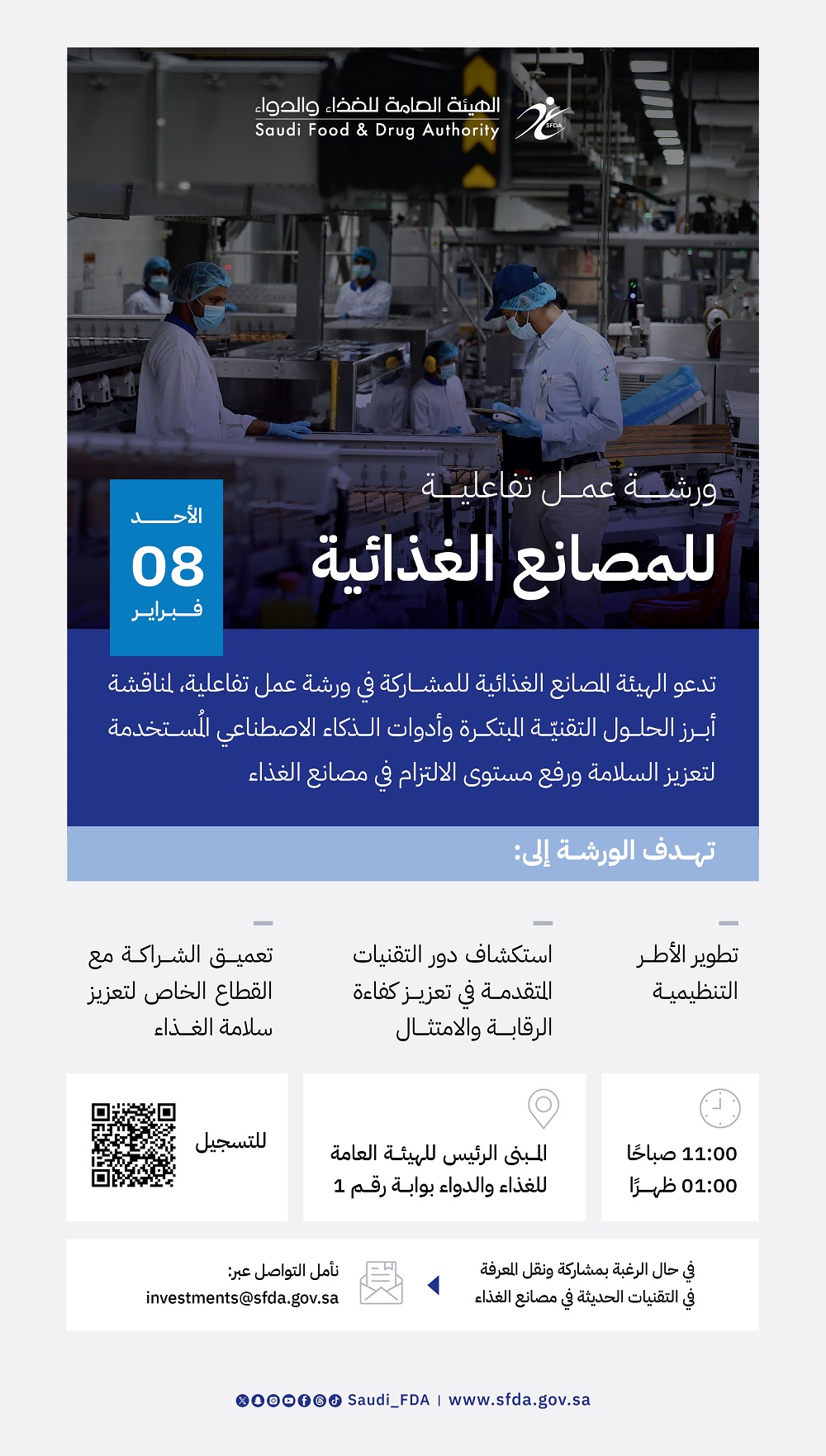
05
NovemberTablet Scoring Compliance: A Regulatory Workshop for Industry
Remotely

Objectives:
" Enhance pharmaceutical manufacturers* understanding of regulatory expectations and best practices for the scientific assessment of tablet subdivision.
• Clarify current guidelines, compliance requirements, and the scientific principles that support robust subdivision evaluation.
* Ensure product quality, safeguard patient safety, and facilitate successful regulatory approval through effective subdivision assessment practices.
| من |
|
| حتى |
|
| نوع الورشة |
عامة
|
| لغة العرض |
الإنجليزية
|
انتهى وقت ورشة العمل