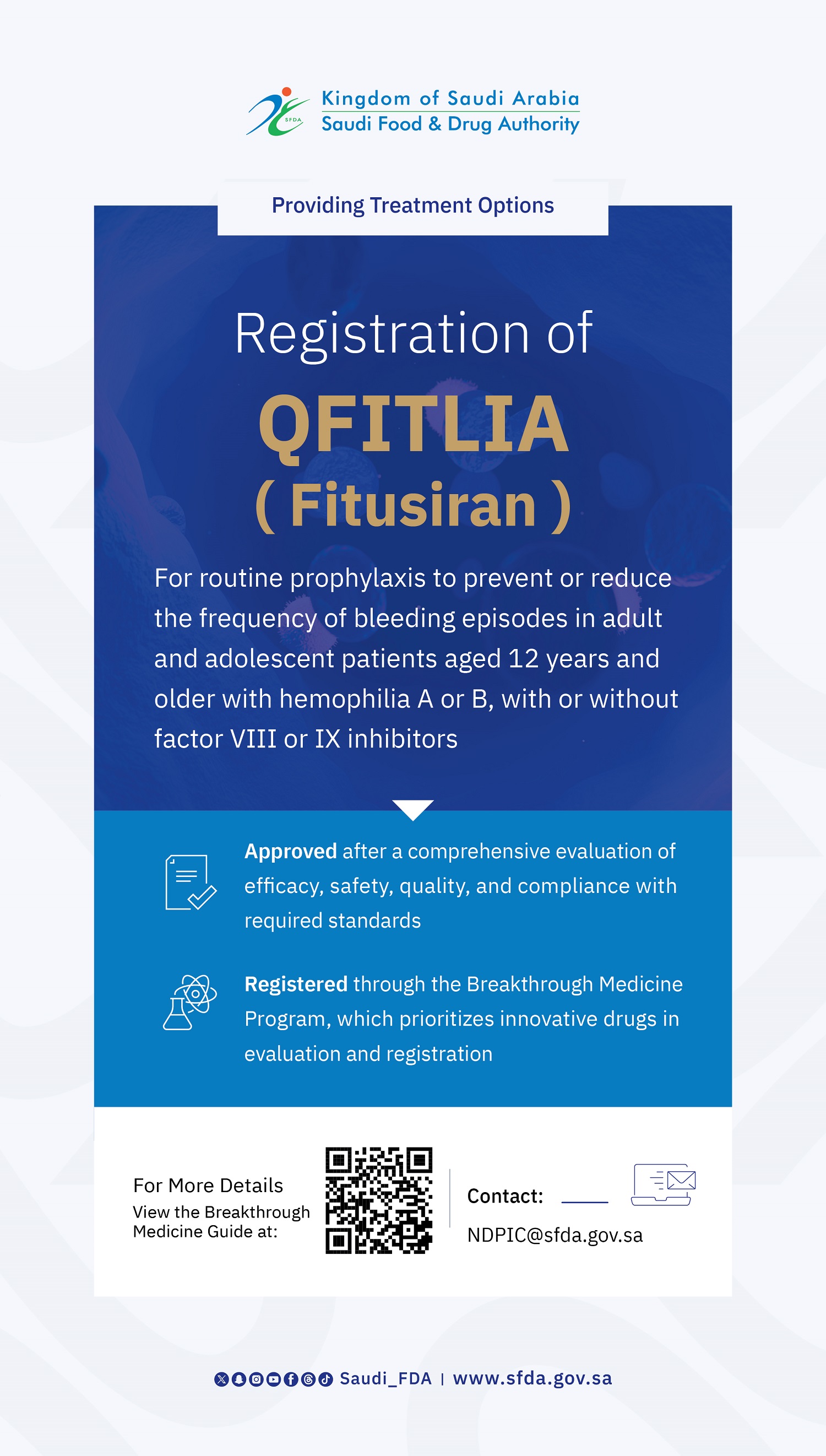
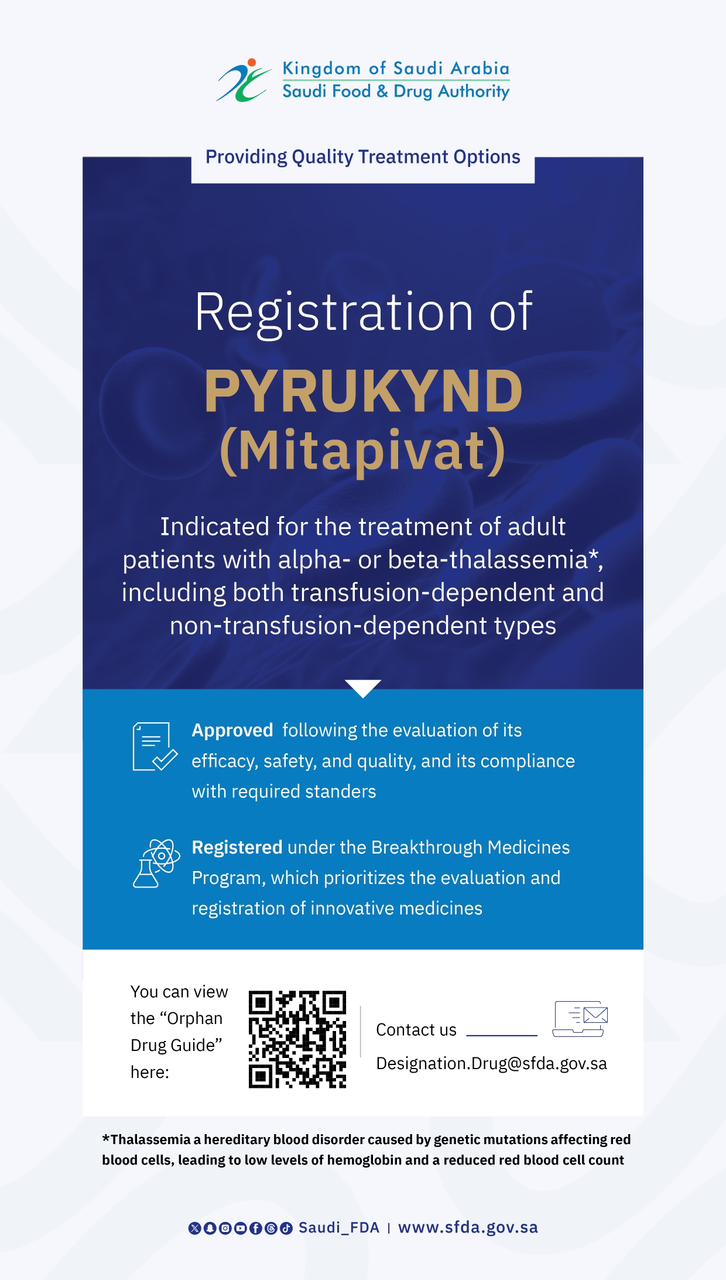
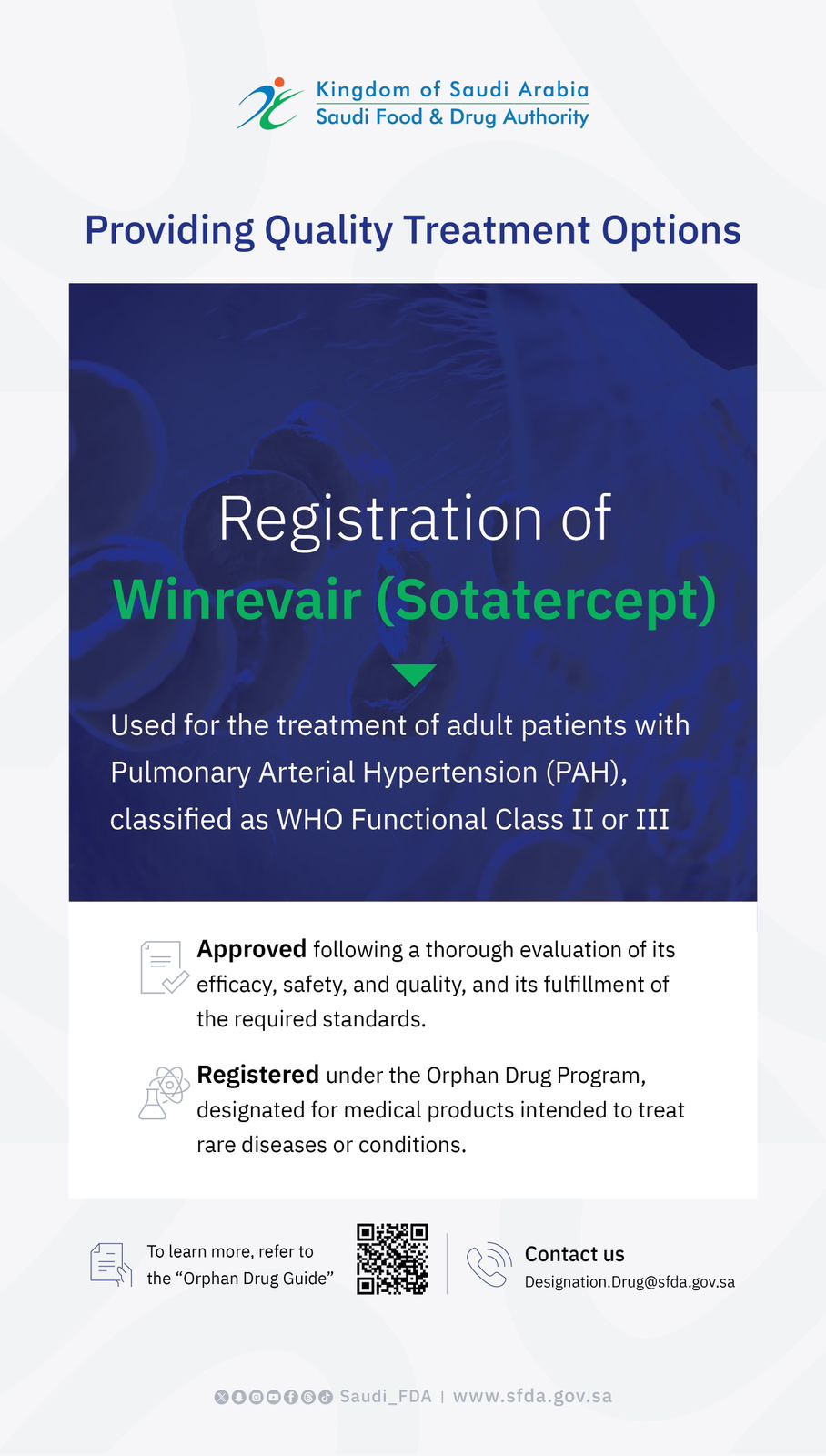
The SFDA Approves Clinical Trial for Novel Biologic Therapy for Genetic Propionic Acidemia
2025-05-04
Experimental Treatment by mRNA
The Saudi Food and Drug Authority (SFDA) has approved a clinical study registration titled "A Global Phase 1/2 Open-Label Dose-Optimization Study to Evaluate the Safety and Pharmacokinetics of the Biologic Treatment (mRNA-3927) in Participants with Propionic Acidemia." This research will investigate an innovative experimental therapy utilizing messenger RNA (mRNA) technology to address a rare inherited disorder affecting amino acid metabolism.
Understanding the Rare Metabolic Condition
Propionic Acidemia is identified as an inherited metabolic condition arising from a recessive genetic defect. This defect results in a deficiency of crucial enzymes required for converting specific amino acids into energy. Consequently, harmful organic acids build up in the bloodstream, leading to acute symptoms such as lethargy, vomiting, and respiratory distress. Without prompt therapeutic intervention, complications can escalate to neurological impairment or organ failure. The Ministry of Health proactively monitors this and other genetic disorders through a newborn screening program, a key component of efforts to decrease disability and mortality, currently encompassing various genetic conditions, including organic acid disorders like Propionic Acidemia.
Treatment Mechanism and Objectives
The investigational treatment, developed by Moderna, involves intravenous administration using lipid nanoparticle technology to deliver messenger RNA to liver cells. This mRNA carries the genetic instructions to produce two protein subunits (PCCA and PCCB), which are essential components of the enzyme (PCC) that is either inactive or absent in individuals with Propionic Acidemia. The goal of this therapy is to enable the body to internally generate the necessary enzyme, thereby restoring normal functional activity and reducing the accumulation of toxic organic acids, ultimately improving the patient's metabolic balance. This approach represents a leading-edge application of mRNA technology in compensating for missing enzymes.
Saudi Arabia Advances Innovation in Healthcare
The SFDA underscores that this approval reflects its dedication to fostering clinical research and attracting new scientific advancements, particularly in the realm of rare diseases. This is facilitated through an efficient and transparent regulatory framework designed to expedite access to promising treatments. This action also aligns with the objectives of the Health Sector Transformation Program, a key initiative of Saudi Vision 2030, which aims to establish the Kingdom as a prominent regional hub for research, development, and health innovation.
For a comprehensive list of registered clinical studies, please visit the SFDA's website: https://www.sfda.gov.sa/en/drug_clinical_trials_list