The SFDA Approves the Registration of “Winrevair” for the Treatment of Pulmonary Arterial Hypertension under the Orphan Drug Designation Pathway
2025-07-10
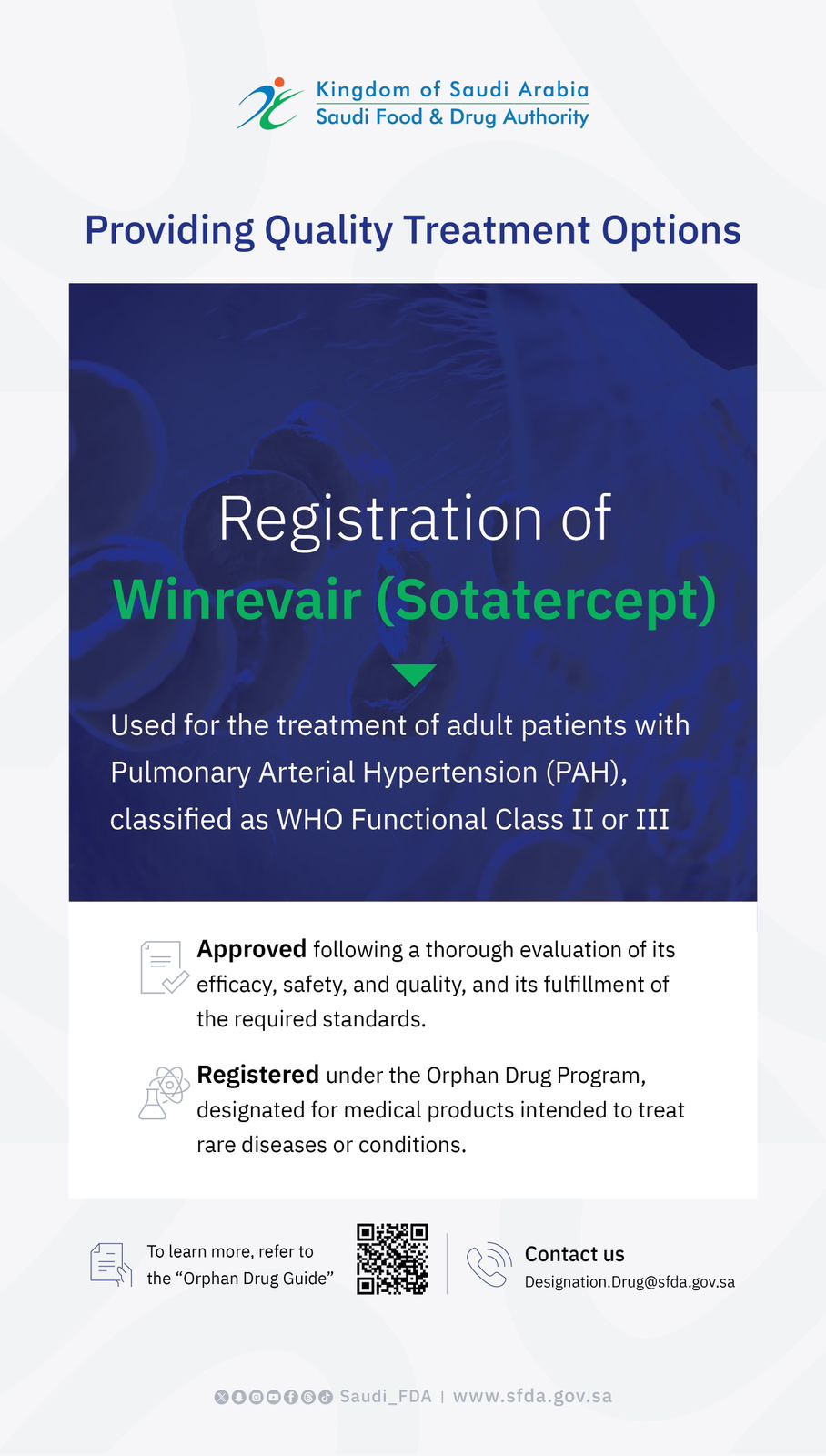
The Saudi Food and Drug Authority (SFDA) has approved the registration of Winrevair (Sotatercept), which has been designated as an orphan drug under the SFDA Orphan Drug Program, for the treatment of pulmonary arterial hypertension (PAH) in adult patients classified as WHO Functional Class (FC) II to III, with the aim of improving exercise capacity.
Life-threatening Disease
PAH is a life-threatening condition characterized by the thickening of blood vessels in the lungs, which leads to increased pressure in the pulmonary arteries. Common symptoms include shortness of breath, chest pain, and fatigue.
An Innovative Mechanism Targeting Activin
Sotatercept works through a novel mechanism by inhibiting activin in the body, a protein that plays a role in increasing the thickness of the blood vessels in the lungs and raising the blood pressure. Inhibiting activin results in improved blood flow in the lungs and decreased pressure, which enhances the physical activity capacity and provides a better quality of life for the patient.
Clinically Significant Outcomes in Reducing Risk of Deterioration and Death
The SFDA confirmed that Sotatercept was approved following a rigorous evaluation of its efficacy, safety, in accordance with applicable regulatory standards. Clinical studies demonstrated a statistically significant reduction in the risk of disease progression and death in PAH patients who classified as WHO Functional Class II or III. Furthermore, data showed an acceptable safety profile. The most common side effects observed were headache, nosebleeds, and skin itching.
The SFDA Orphan Drug Designation Program Accelerates Access to Critical Therapies
The SFDA committed to facilitating access to effective therapies, particularly for rare and hard-to-treat diseases, which often lack sufficient treatment options. In line with this commitment, the SFDA launched the Orphan Drug Program, which serves as a strategic pillar to promote pharmaceutical innovation and address unmet medical needs, supporting the objectives of Saudi Vision 2030 to enhance the quality of healthcare.
An orphan drug is defined as a medication intended to treat a rare disease or condition that affects fewer than five individuals per 10,000 people in the Kingdom of Saudi Arabia.
This approval reflects the SFDA’s continued commitment to supporting innovation, enhancing treatment accessibility, and ensuring the safety and effectiveness of therapies provided to patients across the Kingdom.
For further information about the Orphan Drugs Guideline, please refer to the guide available on the SFDA website: https://sfda.gov.sa/en/regulations/89310 or contact the SFDA via email at Designation.Drug@sfda.gov.sa