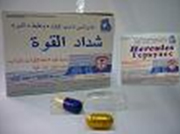
SFDA Warns about Two Herbal Products Used as Sexual Capability Promoters
2011-02-12
In view of its role of monitoring and control of safety and security of drug and herbal products ; SFDA recalled two herbal products from the local market which are promoted and alleged to increase sexual capability.
Analysis of the tow products revealed that they are counterfeited by addition of high concentrations of Sildenafi, the active agent in "Viagra®" product, and are exceeding the recommended dose that must be used under medical supervision, due to the serious side effects that may lead to death, especially in elder people, patients with heart disease and patients with high blood pressure.
Whereas these herbal products are not registered within SFDA, and whereas the activity of the promoting company is considered a commercial counterfeit and misleading consumes by promoting these products as natural products; while their source or extent of safety , security , content and quality level is unknown, and despite of their adverse impact on health and serious side effects that may lead to death, especially in elder people, patients with heart disease and patients with high blood pressure.
Therefore, trading of these products is considered an explicit violation to the ‘Pharmaceutical Organizations & Products Regulations’, issued by the Royal Decree No. M/31 dated 1/6/1425H and its implementation bylaws. Accordingly, SFDA has addressed the concerned authorities to take the necessary actions to recall and ban selling of these products in the local markets and take the necessary disciplinary actions towards any entity selling or marketing these products
Moreover, SFDA advises consumers not to be mislead by such false alleges, which are absolutely untrue and may be harmful to the users of these products.