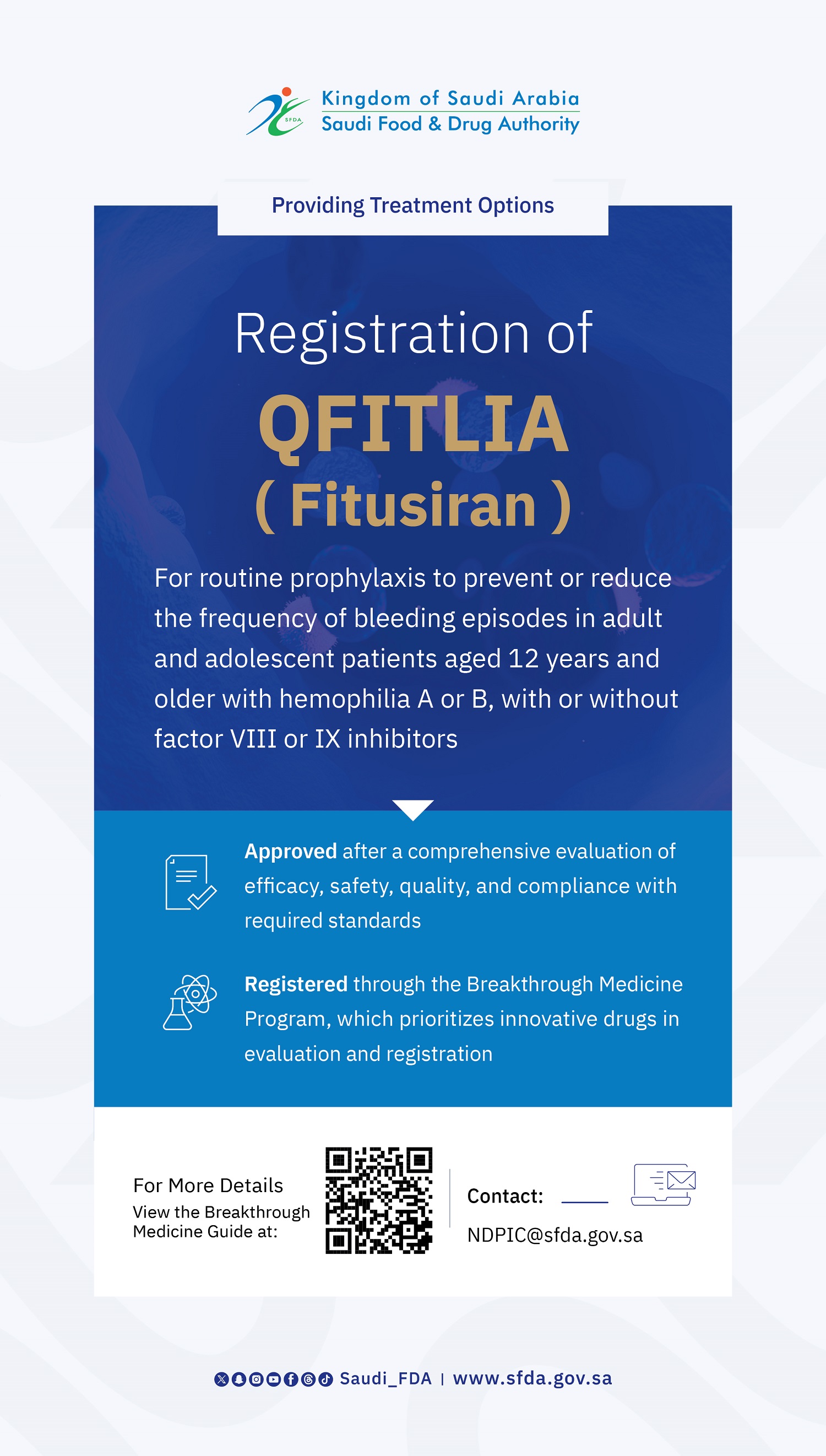
The SFDA Grants Breakthrough Designation to “MIQNAF” for Acute Bacterial Infections Treatment
2025-05-22
The Saudi Food and Drug Authority (SFDA) has granted Breakthrough Designation to MIQNAF (Nafithromycin), developed by Wockhardt Bio AG. This designation is for its use in the treatment of adults with community-acquired bacterial pneumonia (CABP) and other mild to moderate infections such as acute bacterial exacerbations of chronic bronchitis (AECB), acute bacterial sinusitis, and pharyngitis/tonsillitis caused by susceptible pathogens.
First Macrolide Antibiotic in 30 Years with Promising Efficacy
Nafithromycin represents the first new macrolide antibiotic developed in the past 30 years, showing promising efficacy and safety in clinical trials, specifically to combat bacterial infections caused by strains resistant to currently available treatments.
Promising Safety and Efficacy Profile
This breakthrough designation was based on positive results from clinical studies. These studies showed effective treatment of community-acquired bacterial pneumonia in adults, with no serious side effects compared to the comparator antibiotic. The findings suggest that Nafithromycin could offer a new treatment option for patients with antibiotic resistance and limited effective alternatives.
Registration is Subjected to Full Scientific Evaluation
The SFDA emphasizes that this designation allows MIQNAF to be submitted and reviewed under the Breakthrough Track, subject to specific regulatory controls and mechanisms. This designation does not constitute a marketing authorization of the drug in Saudi Arabia. A final decision regarding registration will be announced following a full technical and scientific evaluation of the complete registration dossier.
The SFDA Breakthrough Medicine Program Expedites Access to Targeted Treatments
The Program aims to expedite access to innovative and effective treatments for serious diseases where existing therapies may be inadequate. To qualify for this designation, a drug must have completed clinical trials demonstrating promising efficacy and safety, offer a significant therapeutic advantage over current options, target serious or life-threatening conditions, and exhibit a positive benefit-risk balance. Notably, the product should not be registered with any other regulatory authority at the time of application.
For further information about the Breakthrough Medicines Program, please refer to the guide available on the SFDA website:
https://sfda.gov.sa/en/regulations/89310
or contact the SFDA via email at Designation.Drug@sfda.gov.s