Drugs
Drugs
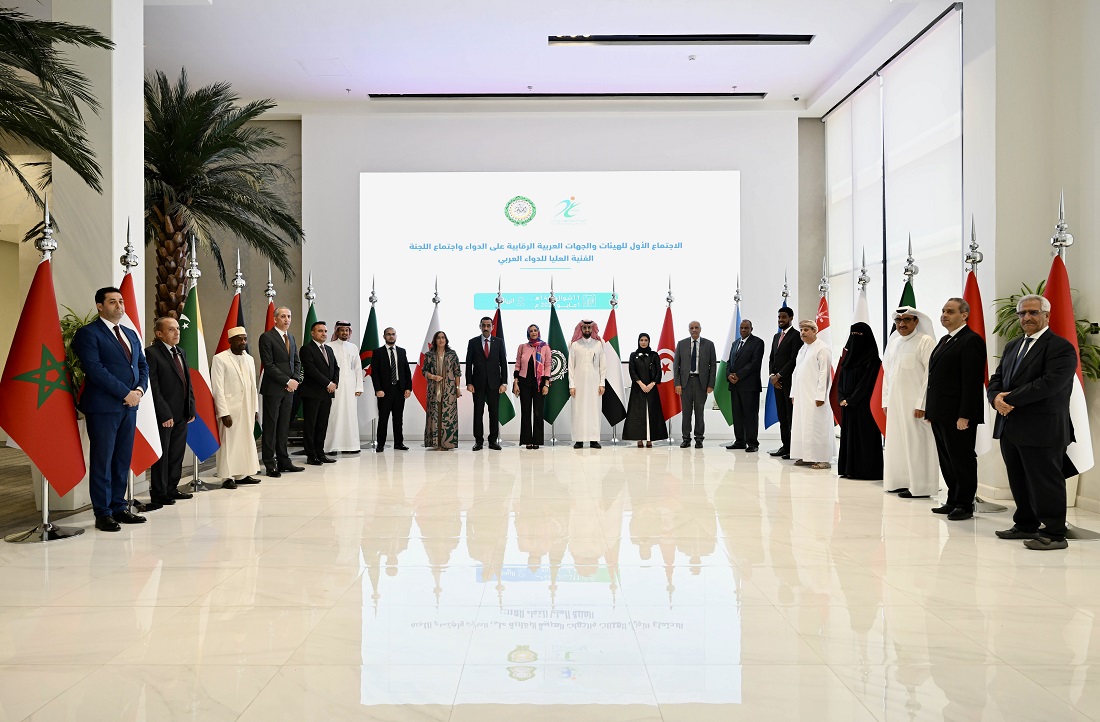
Saudi Arabia Hosts 1st Meeting of Arab Authorities Controlling Medicines
2023-05-01
The Kingdom of Saudi Arabia, represented by the Saudi Food and Drug Authority (SFDA), hosted the 1st Meeting of Arab Authorities Controlling Medicines today at the authority’s headquarters in Riyadh.
This meeting comes in implementation of Resolution No. (11) of the Council of Arab Ministers of Health in its (56th) regular session for 2022, as the Kingdom of Saudi Arabia welcomed at that time to host the aforementioned meeting, which is the first of its kind at the Arab level.
Other News
Specifications: Test Procedures and Acceptance Criteria for New Veterinary Drug Substances and New Medicinal Products: Chemical Substances (VICH GL39)
2023-03-29SFDA CEO inaugurates DIA Middle East Conference
2023-02-13
His Excellency the CEO of the Saudi Food and Drug Authority (SFDA), Dr. Hisham bin Saad Aljadhey, inaugurated the first Drug Information Association (DIA) Middle East Conference in Riyadh, which will be from 13 to 14 February 2023 in partnership with the SFDA.
Other News
SFDA: SDR System Records Great Numbers Since Launching
2023-01-18
Saudi Food & Drug Authority (SFDA) records more than 30,000 applications via Saudi Drug Registration (SDR) System since the launch of its new version in mid-2021. SDR registered more than 8,000 medicinal, herbal and veterinary products, and issued more than 8,000 certificates.
Other News
SFDA: 45 Violating Facilities in December
2023-01-08
Saudi Food and Drug Authority (SFDA) penalized 45 facilities during December 2022, due to lack of commitment to provide the registered pharmaceutical products to the local market, inform instantly to the movement of the drug in the electronic tracking system, and report on expected shortage or interruption in the supply of pharmaceutical products that have been registered with the Authority.
Other News
- Previous page
- Page 14
- Next page