“SFDA” Warns Consumers against using two Makeup Products due to Contain High Levels of Lead and Arsenic
2021-09-27
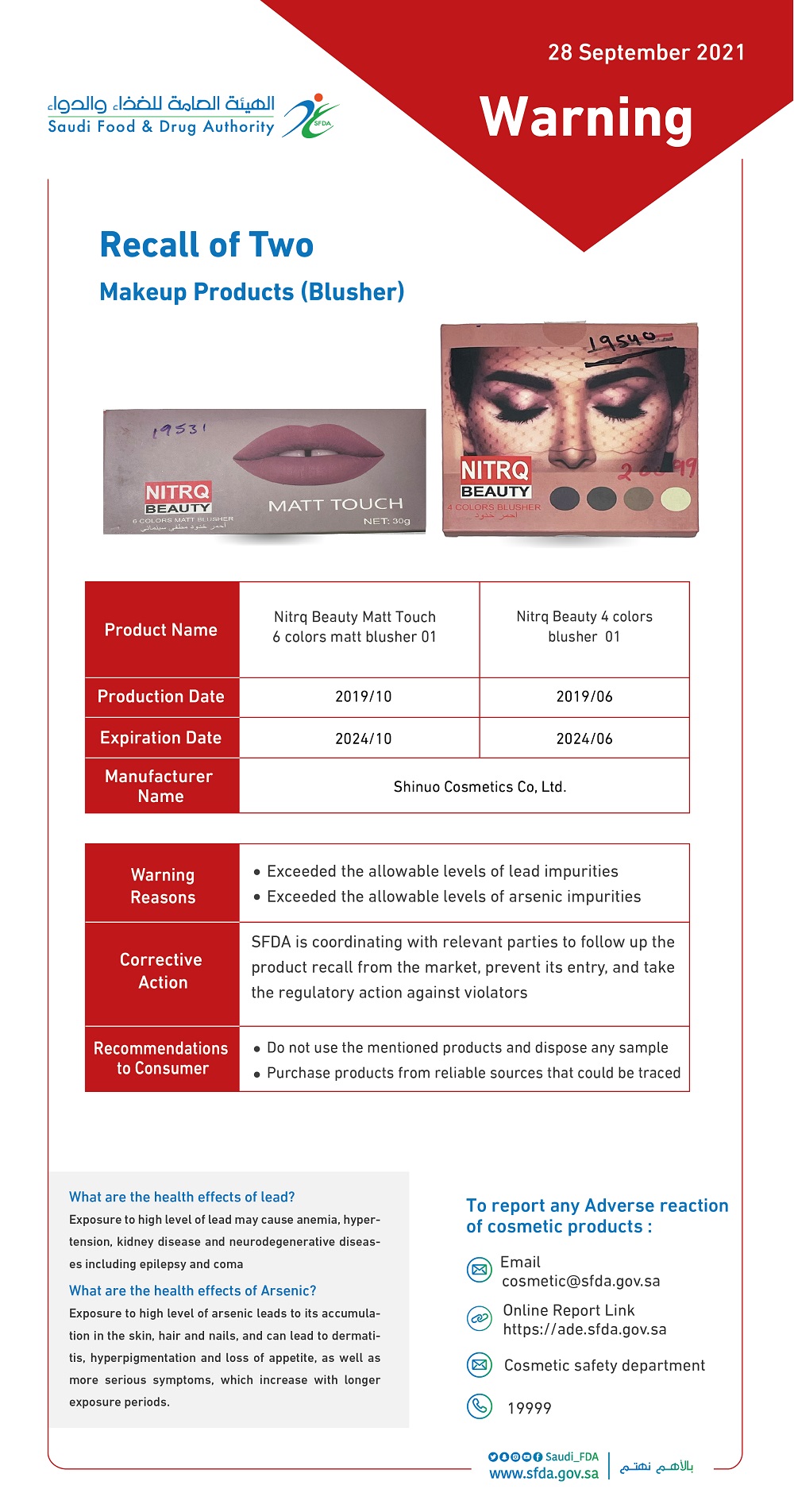
The Saudi Food and Drug Authority (SFDA) issued warning against the use of two "blush" makeup products as they contain high level of lead and arsenic, which exceeded the acceptable impurity limits according to the safety requirements of cosmetics and personal care products regulations NO. SFDA.CO/GSO 1943:2016.
Noting, exposure to high level of these substances may cause health risks to the consumer.
Products details are as follow:
• "Nitrq Beauty 4 colors blusher 01", with an expiration date of 06/2024
• "Nitrq Beauty Matt Touch 6 colors matt blusher 01" with an expiration date of 10/2024
The authority indicated that exposure to high level of lead may cause anemia, hypertension, kidney disease and neurodegenerative diseases, while exposure to high level of arsenic leads to its accumulation in the skin, hair and nails, and can lead to dermatitis, hyperpigmentation and loss of appetite, as well as more serious symptoms, which increase with longer exposure periods.
The SFDA advised consumers not to use the mentioned products and dispose any sample they may have, emphasizing the importance of purchasing products from reliable sources.
The SFDA pointed out that it is taking the necessary actions in cooperation with relevant parties in order to follow up the product recall from the markets, preventing its entry, and taking the regulatory action against violators.