Post-market evaluation for the safety of hemodialysis devices related VND and ABLS
Post-market evaluation for the safety of hemodialysis devices related VND and ABLS
The process of hemodialysis is mainly about filtering the patient’s blood through an artificial kidney outside the patient’s body called dialyzer by series steps as explained in figure 1 below [1]. During hemodialysis session, there are two bloodline access needs to be connected between patients and the hemodialysis device. One of the tubes (red bloodline) is function to transport blood from patient’s body into the machine. Then, the blood access the pressure monitors and pumps to maintain the desired rate level of the blood flow. After that, the blood flow into the dialyzer to start the process of filtration. Upon entering the dialyzer, the blood is filtered by a Dialysate solution that pulls the waste out and balancing electrolytes of the blood. Following that, blood enter pressure monitor and air trap to ensure the safety of blood to transfer back to the patient’s body. In final, the filtered blood flow back to the patients’ blood though the second tube (gray bloodline) [1].
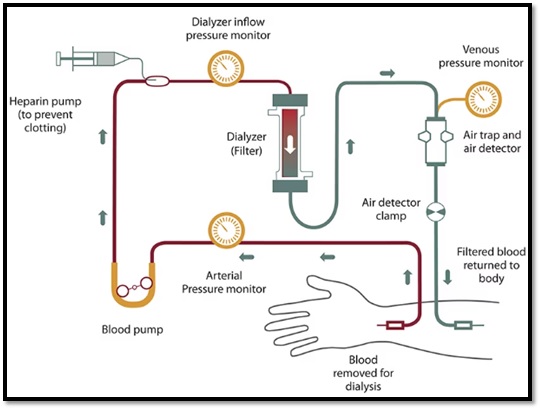
Figure 1: hemodialysis process [2].
Before starting hemodialysis, creating vascular access is an essential step. The vascular access is the source of connection to dialyzer, where two needles is placed in patient’s body to allow the exchange of blood during dialysis. There are three different types of vascular access, which are arteriovenous (AV) fistula, AV graft, and catheter [2].
To construct an AV fistula, healthcare provider joins an artery to a vein, generally in patients arm as shown in figure 2. The main purpose of this connection is making the vein wider in order to filter the maximum amount of blood through dialysis process [3].
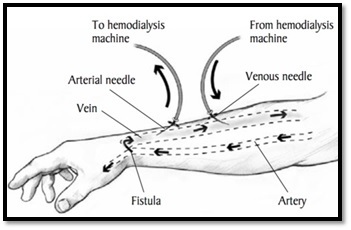
Figure 2: arteriovenous (AV) fistula [2].
However, if healthcare provider unable to create an AV fistula due to vein difficulties, patient may require an AV graft instead. AV graft is soft tube to link an artery to a vein that construct an AV graft as illustrated in figure 3 [3].
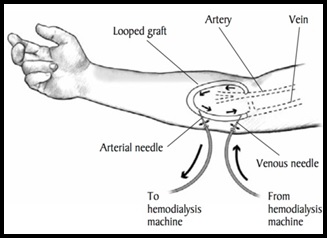
Figure 3: arteriovenous (AV) graft [2].
The last type is venous catheter access, which is tube inserted into a vein either in patient’s neck, chest, or leg. Groin, considered temporary access. This vascular access is required when the kidney failure has advanced rapidly [2].
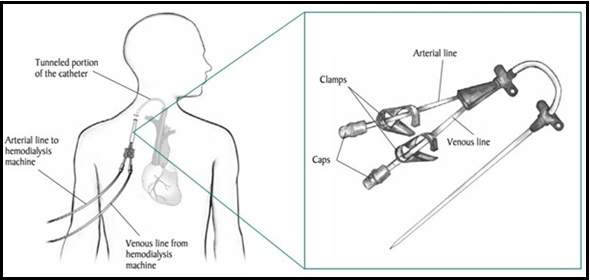
Figure 4: venous catheter access [4].
The safety of hemodialysis has been reported extensively in several scientific articles due to unexpected risks that associate with this device. The potential risk associated with hemodialysis procedure is classified as a life-threatening health hazards, which is a dislodged venous needle or a catheter. Needle dislodgment (ND) is caused by the disconnection of the needle from the vascular access either venous (VND) or artery (AND) needles; however, access bloodline separation (ABLS) is caused by detachment of the central venous catheter (CVC) or fistula needle from the hemodialysis bloodline circuits [5]. Hemodialysis typically filtered blood at about 500 mL/minute, so any needle dislodgement during the procedure can result in life-threatening events within minutes [5].
Therefore, it is highlighted as the fourth health technology hazard of the top 10 health technology hazards reported in 2023 by ECRI followed by published several cases in the global medical literature about the issue. It specified that there is a danger of undetected VND or ABLS events during hemodialysis, which lead to severe injury or death [5].
Purpose
This study examined and highlighted the potential risks of VND and ABLS while using hemodialysis devices in light of their adverse events and complaints, risk factors, complications, protective recommendations, reporting awareness within the Saudi healthcare providers.
Scope
The scope of this report was to evaluate the safety of hemodialysis devices by using a survey-based approach, which gather the real-world data from Saudi healthcare providers regarding the VND and ABLS. The deliverables will be demonstrated in the form of current-evidence-based recommendations, which derived using appropriate approaches in the data collection and analysis regarding the safety of hemodialysis as a technique and not limited for specific brands.
Literature Review
In order to utilize medical devices safely, it is essential to identify possible risks associated with those devices and to minimize or eliminate the risk of adverse events. Therefore, researchers are assisting in informing healthcare communities, biomedical management, medical device manufacturers, and medical device regulators by publishing medical literature about those issues. In fact, published literature can play an essential role to encourage healthcare communities and societies to develop guidelines that specify the risk characteristics, risk assessment, and precautions to prevent such adverse events.
Back in 2012, the American nephrology nurses association (ANNA) assigned a task force to highlight the risk of arteriovenous needle dislodgment during hemodialysis sessions. They investigated and reported information related to VND including, the adverse events and complaints, risk assessment, risk intervention, and recommendations to raise the awareness of healthcare providers and eliminate its occurrence [6].
While in 2021, VND topic was reviewed by ANNA to determine if the 2012 practice recommendations should be updated by reviewing the current literature and expertise in the field. As a consequence, another task force was established in order to provide updated evidence-based information and practices that may help to reduce the risk associated with VND and ABLS while patient on hemodialysis [7].
In 2023, ECRI reported that there are potential life-threatening adverse events that can occur while using hemodialysis. For instance, blood loss leads to either severe injury or mortality. Such events can appear due to the dislodgment of an arteriovenous needle from the attached vascular access or venous catheter from the attached bloodlines. Not only, that, but ECRI also listed the risk factors that might increase the occurrence of VND and ABLS, and protective measures to decrease the chance of appearance [5].
Over several years, the amount of data has accumulated and experts have highlighted the consequences of VND and ABLS, which then revealed that it is life-threatening issues
Part I: Methodology
Numerous reports were published globally since 2012 to explain the potential risk of VND and ABLS during hemodialysis session, while the local status of this issue was unknown. Therefore, this clinical evaluation was conducted to reveal the potential risk of VND and ABLS associated with hemodialysis devices within Saudi healthcare providers.
The first domain of the study was to extract information related to VND and ABLS in order to construct a questionnaire. While the second domain is to use this questionnaire to cumulate information of VND and ABLS while using hemodialysis devices in light of their adverse events and complaints, risk factors, complications, protective recommendations, and reporting awareness.
Accordingly, a computer based self-administrated questionnaire was constructed to be used as data collection method to obtain all the required data from Saudi experts in the nephrology field.
The sampling technique used to obtain the sample size is a convenience sample. The sample contained 80 Saudi healthcare providers (HCP) and 1 specialized society that were requested to fulfill the required questionnaire.
In order obtain the required information, the questionnaire was segregated into two main sections: the first part covers 5 questions about the VND and ABLS in relation to dialysis sessions, while the second part contains 1 specific question about the governance awareness in reporting complications. These questions structured by various format including open-ended, close- ended along with conditional questions.
Part II: Results
Saudi healthcare providers were consulted to provide the surveillance data regarding the safety of hemodialysis related VND and ABLS. During the analysis, 40 entrants participated in the survey from August to October 2023, 22 of whom completed the survey. The response rate is 55% (22 out of 40). Thus, the analysis process executed on the completed responses according to the following subsections.
Figure 5: HCP demographic and the type of hemodialysis service.
The initial two elements of the survey listed the demographics of HCPs within the Saudi healthcare providers, who filled out the questionnaire. Among 22 HCPs, 82% are working in nephrology ward, while 18% are practicing their profession in other department within the healthcare facility as shown in figure 5 below.

Figure 5: HCP demographic and the type of hemodialysis service.
II.II Adverse events and complaints
Another two elements were designed to reveal the adverse events and complaints related to the use of hemodialysis machines. Figure 6 demonstrates the responses of 18 nephrology nurses, 44% of them stated that they faced a total of 33 adverse events and complaints during hemodialysis sessions. For instance, 24 cases of VND, 7 cases of ABLS, 1 case of mild hypotension, and 1 case of clotted circuit. Whereas, 56% of the nurses declared that there is no risk to be identified with the use of hemodialysis machines.

Figure 6: incidents associated to hemodialysis machines
As a result of the adverse events and complaints associated with hemodialysis machines, several further complications have arisen that threaten the patient's life. Among the 44% of the nephrology nurses, who reported the above adverse events and complaints, disclose that VND and ABLS led to 31 cases of slight blood loss, 1 case of Hypovolemic shock, and 1 case of Mild hypotension.
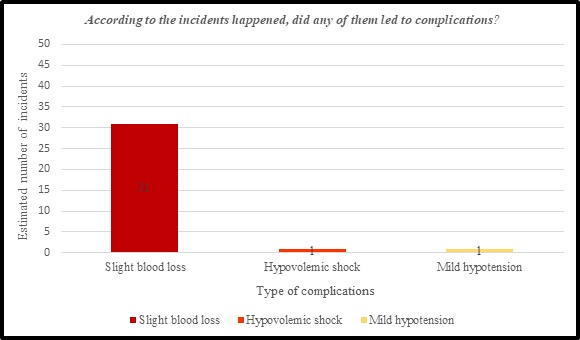
Figure 7: complication associated with THE INCIDENTS of hemodialysis machines
Bases on the adverse events and complaints reported, it is noticeable from figure 9 that 44% of nephrology nurses concur on two main risk factors responsible for the adverse events and complaints by 50 % among all risk factors. These two factors were attributed to “patients suffered from altered mental states, such as restless or not fully conscious” and “patients experienced treatment complications, such as Hypotension or muscle cramps”. While other factors, such as “Catheter disconnection bleed”, “patients with difficult cannulation” and “patients refused to keep access and bloodlines uncovered” were considered as lower risk factors. Whereas, the “patients dialyzing at home” and “taping technique” are not counted as responsible factors for the adverse events and complaints associated with hemodialysis machines as explained in figure 8.
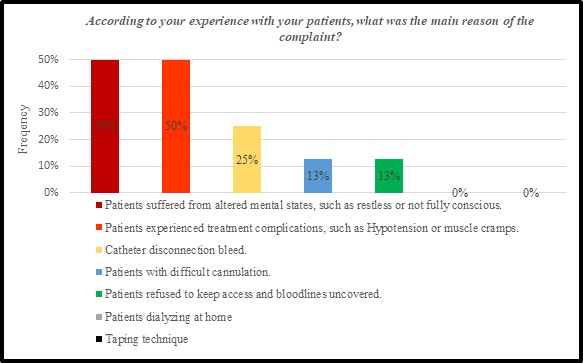
Figure 8: Risk factors of VND and ABLS.
II. V Preventive recommendations
One of the questionnaire elements chose to reveal the protective factors that decrease or prevent the likelihood of patient to develop VND and ABLS. 82% of the nephrology nurses chose 3 factors, which are “practice to implement taping technique”, “educate the patients, specifically in keeping the vascular access visible”, and “educate Staff to observe and examine of patient access and bloodlines”, illustrated that they are the most effective preventive measures. Following that are “practice to secure Bloodlines”, “educate the patients by providing patient tool to articulate the risk and educate patients in risk reduction”, and “obtain well operation training from the manufacture or certified personnel to recognize the ability and the limitations of a hemodialysis machines to maintain the safety and eliminate the risk”. Moreover, “use Blood loss detector” was reported as the least factors that can minimize the occurrence of VND and ABLS. Whereas, the “Strict monitoring during and post dialysis” is provided as an additional recommendation to decrease or prevent the adverse events and complaints associated with hemodialysis machines as showed in figure 9.
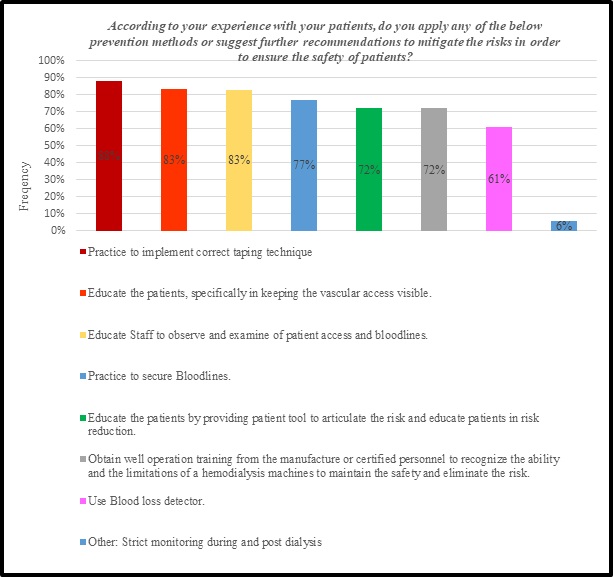
Figure 9: PREVENTION METHODS OF VNS AND ABLS.
One element measured to disclose the realization of the healthcare community regarding the importance of Reporting complaints related to medical devices, which helps in taking immediate corrective action, elevating the awareness of the potential risks of the device, and the way to prevent reoccurrences. Nevertheless, figure 10, and as revealed by the respondents, demonstrate that 13 % of nephrology nurses always report complaints related to hemodialysis machines, while 88% of them never reported any incidence related to hemodialysis to the National Centre for Medical Devices Reporting (NCMDR) in SFDA, which reflect that they are not fully aware about the regulatory practice.

Figure 10: the awareness level of the HCP in reporting to NCMDR
Part III: Conclusion
This clinical study was initiated to evaluate the risk of VND and ABLS associated with dialysis devices in terms of the following adverse events and complaints, complications, risk factors, preventive recommendations, and reporting awareness among Saudi healthcare providers. Moreover, to examine the level of healthcare providers’ knowledge about the association of VND and ABLS with dialysis devices.
After analyzing the data, several significant findings were detected in regard to VND and ABLS while using dialysis machines within Saudi healthcare providers. 44% of the nephrology nurses who filled out the questionnaire reported adverse events and complaints of 24 VND cases, 7 ABLS cases, and 2 other complaints that occurred during hemodialysis sessions. Those 33 complaints resulted in 31 complications, including slight blood loss, Hypovolemic shock, and Mild hypotension. After following such complaints, nephrology nurses declared two risk factors were the most responsible factors for these complaints: “patients suffered from altered mental states” and “patients experienced treatment complications”. Thereupon, 82% of the nephrology nurses applied 3 factors more than others that explain that they are the most effective preventive measures: “practice to implement taping technique”, “educate the patients, specifically in keeping the vascular access visible”, and “educate Staff to observe and examine of patient access and bloodlines”. Finally, the reporting awareness factor showed an underreporting of 88% by nephrology nurses to report any incidence related to hemodialysis to the SFDA NCMDR.
The questionnaire is a common approach to studying specific trends or experts’ opinions; however, it has some limitations, which may yield numerous discrepancies. First, was missing data, as the survey was distributed electronically to 80 HCPs and 1 society. Second was the response rate from the total number who entered the survey: 55% (22 out of 40) and none from the society. Third was the low generalizability of data, as 80 selected sample size of HCPs was not representative of all HCPs within Saudi Arabia. Not only that, but the sampling technique that was used is a convenient sample, which is characterized by the inability to generalize the results of the survey to the population as a whole.
The current evidence highlights the potential risks of VND and ABLS while using hemodialysis machines; therefore, SFDA would like to advise healthcare practitioners to the following recommendations to restrict their risk prevalence: Healthcare providers must follow guideline that contains the following practices: risk assessment, risk reduction providers to follow, Dialysis units/ centers shall develop an awareness material to articulate the risk of VND and ABLS and educate patients in risk prevention, Hemodialysis manufacturers must provide well operation training to dialysis units/ centers to recognize the ability and the limitations of a hemodialysis machines to maintain the safety and eliminate the risk of VND and ABLS. SFDA must arrange with local societies to evaluate the current practice of VND and ABLS while using hemodialysis devices. SFDA shall create safety communication for Saudi dialysis units/centers.
In conclusion, dialysis machine is one of the life-saving devices that extend patients' lives and improve their quality of life; however, it can pose serious threats if used improperly. Consequently, various recommendations need to be implemented to avoid adverse events and complaints and associated complications, to unify the practice and prevention method, and to elevate the awareness level in reporting adverse events and complaints.
Thanks to the post-market clinical evaluation team for their supports in conducting this work. For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
1. Fresenius kidney care. Hemodialysis Machine. https://www.freseniuskidneycare.com/treatment/dialysis/hemodialysis-machine
2. Romancito, G. National Institute of Diabetes and Digestive and Kidney Diseases. (2018). Hemodialysis Retried from: https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/hemodialysis
3. National kidney foundation. Hemodialysis Access. Retried from: https://www.kidney.org/atoz/content/hemoaccess#how-your-access-works
4. Allon,M. National Institute of Diabetes and Digestive and Kidney Diseases. (2014). Vascular Access for Hemodialysis. Retried from: https://surgery.ucsf.edu/conditions--procedures/vascular-access-for-hemodialysis.aspx
5. ECRI. Top 10 Hazards Executive Brief, 2023. https://www.ecri.org/top-10-health-technology-hazards-2023-executive-brief
6. Axley, B., Speranza-Reid, J., & Williams, H. (2012). Venous needle dislodgement in patients on hemodialysis. Nephrology nursing journal: journal of the American Nephrology Nurses' Association, 39(6), 435–446.
7. Speranza-Reid, J., Brouwer-Maier, D., Cruz, C. M., & Inglese, M. (2021). Venous Needle Dislodgement and Access-Bloodline Separation. Nephrology nursing journal : journal of the American Nephrology Nurses' Association, 48(4), 347–365
8. ECRI. Undetected venous needle dislodgement or access-bloodline separation during hemodialysis can lead to death. Hazard #4—2023 top 10 health technology hazards. Device Evaluation 2023 Jan 11.
9. Ellingson, K. D., Palekar, R. S., Lucero, C. A., Kurkjian, K. M., Chai, S. J., Schlossberg, D. S., Vincenti, D. M., Fink, J. C., Davies-Cole, J. O., Magri, J. M., Arduino, M. J., & Patel, P. R. (2012). Vascular access hemorrhages contribute to deaths among hemodialysis patients. Kidney international, 82(6), 686–692. https://doi.org/10.1038/ki.2012.185.
10. Matos, J. F., Pinto, B., Felix, C., & Peralta, R. (2018). Needle Dislodgement Management in Dialysis—Causes and Consequences. In Genoa, Italy: Paper presented at: 47th EDTNA/ERCA International Conference..
11. Teresa Doyle. (2023). Venous needle dislodgement: information form patient. Oxford University Hospitals.
12. Jose, M. D., Marshall, M. R., Read, G., Lioufas, N., Ling, J., Snelling, P., & Polkinghorne, K. R. (2017). Fatal Dialysis Vascular Access Hemorrhage. American journal of kidney diseases : the official journal of the National Kidney Foundation, 70(4), 570–575. https://doi.org/10.1053/j.ajkd.2017.05.014.


