An Evaluation of the Saudi Healthcare Facilities Compliance to Develop a Procedure for Single-Use Medical Devices
An Evaluation of the Saudi Healthcare Facilities Compliance to Develop a Procedure for Single-Use Medical Devices
Reprocessing single-use medical devices involves regulatory, safety and moral issues that can put patients and healthcare providers at risk of infection or breakdown. Single-use medical devices durability to endure the reprocessing procedure has not been tested or evaluated since it is intended by the manufacturer to be used only once. It is crucial to differentiate between reusable and single-use medical devices, as reusable devices are designed to tolerate repeated disinfection and sterilization procedures.
The compliance of the Saudi healthcare facilities to the SFDA decision of prohibiting the re-use of single use devices has been tested, as revealed by the evaluation of 46 facilities. Additionally, the result was compared to a former study, which was conducted by SFDA in 2014 with a similar purpose, to highlight the progress in the topic over the previous five years [1]. In this study, we aim to test the progress of the Saudi hospitals compliance for developing a single-use medical devices policy and procedure, and to compare the results with the previous results that were obtained in 2014.
Single-use medical devices characteristics and design might be altered during reprocessing, which will affect the functionality, safety and efficacy of the medical device [2]. It is also possible that full decontamination of single-use devices cannot be achieved due to the complex shapes and forms of these devices, which will keep patients and healthcare providers at risk of cross-infections [3]. According to the Joint Commission International (JCI), the following risks might be caused as a result of reprocessing single use devices [4]:
- The characteristics of single-use medical devices will possibly get altered during the reprocessing procedure, which will affect the performance and value of the device leading it to fail to achieve its main purpose.
- Single-use medical devices are not validated nor tested for reuse or reprocess by the manufacturer.
- Some single-use medical devices are made of materials that can absorb the sterilization agents or corrode after the reprocessing procedure.
Some single-use medical devices are made of materials that may fail, break, or stretch when undergo a reprocessing procedure.
Part I: Methodology
A sample of 46 Saudi healthcare facilities were evaluated on-site for having a clear policy and procedures to prevent the re-use of single-use medical devices. The level of compliance will be then compared with previous results of a survey obtained in 2014, to highlight the progress in compliance to the SFDA safe-use requirements that relate to single-use devices.
Part II: Results
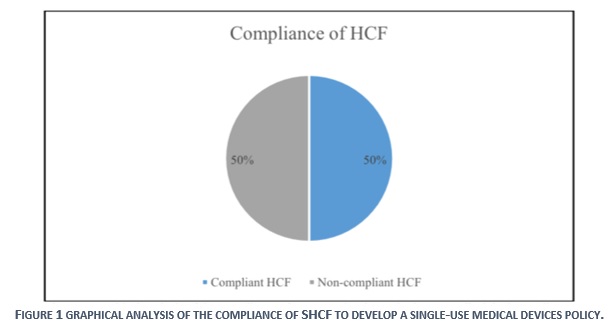
In 2014, SFDA has formed a survey that was distributed over 463 healthcare facilities, to study the situation of applying single-use medical devices policy in KSA. The results revealed that 23% (104) of SHCFs have a written policy regarding the reuse of single-use medical devices. [1] However, the results of evaluation in this study, which was conducted in 2019, indicated a compliance level of 50% (23). Despite the achieved progress of 27% in between 2014-2019, the level of compliance is still very low, as half of the Saudi healthcare facilities are still in none-compliance to the SFDA requirements for the safe-use of single-use devices.
Part III: Conclusion
Despite the fact that reusing single use medical devices lead to serious safety events, this study indicated that nearly half of the Saudi healthcare facilities have not yet developed a clear policy to prevent such a practice. With this in mind, the primary finding of this study comes to suggest doing more efforts to increase the awareness of the risks associated with re-using single-use devices throughout the available channels.
In reference to the findings of this study, the following actions are recommended:
- Raise the awareness of the threats associated with reusing single-use medical devices to patient safety among healthcare providers
- Further efforts are needed to ensure the compliance of Saudi healthcare facilities to the safe-use requirements of single-use medical devices
The following actions were taken by SFDA to ensure the safe-use of single-use medical devices:
- Non-compliance hospitals were directed to follow the safe-use requirements of single-use medical devices, and followed-up to check the status of compliance to develop a clear procedure for preventing re-using single-use medical devices.
- A number of webinars were conducted to increase the awareness of the Saudi healthcare providers of the necessity to prevent the re-use of single-use medical devices.
Grateful thanks to Eng. Danah Alotaibi who designed the study, worked out the data collection and analysis, and wrote up the study context. Eng. Bader Aloufi verified the study methodology and supervised the study progress. With the appreciation to Eng. Ali Masmali for drafting this summary and the post-market clinical evaluation team for their efforts in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
1) Essam Mohammed, Mohammed Alhayan, Abdullah Alangry, Abdullah Aldossari, Aisha Said, Hussien Alshamari, Studying the Reuse of Reprocessed Single Use Medical Devices, Riyadh: Saudi Food and Drug Authority, 2015.
2) Organization, World Health Organization and Pan American Health, Decontamination and Reprocessing of Medical Devices for Health-care Facilities, WHO Library Cataloguing-in-Publication, 2016.
3) Azizi J, Basile RJ, "Doubt and proof: the need to verify the cleaning process.," Biomed Instrum Technol., 2012.
4) Mansur, Jennell M., "Reuse of Single-Use Devices: Understanding Risks and Strantegies for Decision-Making for Health Care Organizations," Joint Commission International, 2017.


