Post Market Clinical Evaluation for the Safety of Endoscopic Devices and Accessories
Post Market Clinical Evaluation for the Safety of Endoscopic Devices and Accessories
Endoscopy procedures play an integral part of the prevention, diagnosis and treatment of gastrointestinal (GI) diseases. However, due to the design complexity of endoscopes, it is difficult to clean and disinfect some inner channels. Thus, bacteria may be able to form biofilms over the inner channel surfaces. Subsequently, contaminated endoscopes could be the starting point of nosocomial infections outbreak. [1]
Despite the accessibility to reprocessing procedures and guidelines, these incidents continue to occur due to multiple causes such as; use of defective equipment, reprocessing failures, or noncompliance with recommended guidelines or manufacturers recommended procedure. [2]
Effective reprocessing of endoscopes includes pre-cleaning, leak testing, manual cleaning, high-level disinfection, rinsing and drying followed by proper storage and documentation. [3] There are different methods and procedures to evaluate the success of the reprocessing process validated by multiple guidelines and organizations.
In this study, a review of international guidelines will be conducted. In addition to an analysis of a previously conducted survey to reach survey-based recommendations that ensure the safety of the current endoscopes reprocessing procedures.
The purpose of this study is to evaluate the risks associated with flexible endoscopes regarding the spread of infections. Based on that, this study aims to assess the level of safety and quality of the current practices of sterilizing endoscopes within Saudi healthcare facilities. In addition, a review of five different global guidelines will be discussed and compared.
This study is specific for evaluating the safety of flexible endoscopes of all brands, as revealed by data collected from Saudi users. The deliverables are specific for this particular product, and will be delivered in form of a survey-based recommendations, which are derived using appropriate statistical approaches in the data collection.
Part I: Methodology
A review of four different international guidelines will be conducted to analyze the difference and compare the recommended procedures. After that an analysis of a previously conducted survey will be presented to derive survey-based recommendations.
International Societies Guidelines for Endoscope Reprocessing
There are several guidelines published by international societies of endoscopic devices and its accessories. In this study, a summary of the recommended best practices of four societies will be presented. The four societies are;
- Centers for Disease Control and Prevention (CDC); Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the Healthcare Infection Control Practices Advisory Committee [4]
- World Gastroenterology Organization (WGO); Endoscope disinfection update: a guide to resource-sensitive reprocessing [5]
- British Society of Gastroenterology (BSG); Guidance on Decontamination of Equipment for Gastrointestinal Endoscopy [6]
- European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroentrology and Endoscopy Nurses and Associates (ESGENA); Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – Update 2018 [7]
Questionnaire
In 2021, the risk Analysis Team surveyed Saudi healthcare facilities to evaluate the safety of current practices. (See Appendix 1) The questionnaire covered four main elements:
- General Questions
- Reprocessing Procedure Questions
- Adverse Events Questions
- Suggestions
Our main focus in this study will be over the questions related to the most common recommended practices.
Part II: Results
In this part of the study, a comparison of global endoscope sterilizing guidelines will be presented. Also, an analysis of the responses of 17 flexible endoscopes users to address the essential concerns found in the survey.
International Societies Guidelines for Endoscope Reprocessing
In this section, a summary of the guidelines of international societies and medical associations regarding reprocessing protocols and best practices to avoid the transmission of infections. In addition to a summary of common recommended practices among different guidelines and recommendations. See table 1.
In 2017, the Centers for Disease Control and Prevention (CDC) published a guideline on essential elements of reprocessing program for flexible endoscopes. The guideline included a list of recommendations on how to build a reliable, high-quality reprocessing system for endoscopes.
World Gastroenterology Organisation (WGO) is a federation of 119 member societies of gastroenterology, hepatology, endoscopy, and other related disciplines. In 2019, WGO published a guide to resource-sensitive reprocessing, which includes endoscope disinfection best practices.
The British Society of Gastroenterology (BSG) is focused on the promotion of gastroenterology and hepatology. In 2020, BSG published a guidance on decontamination of equipment for gastrointestinal endoscopy. The guidance included a set of recommendations for healthcare staff to follow.
European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) published a position statement in 2018, to clarify the best practices of reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy.
Table 1. Reviewed guidelines and their corresponding organizations.

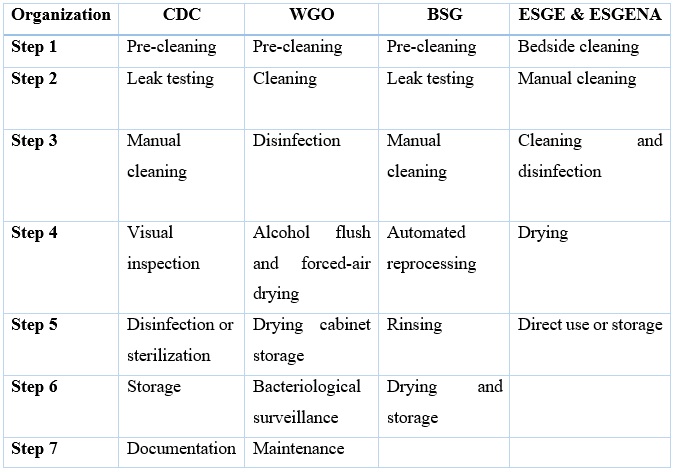
The review of the previously mentioned guidelines resulted in table 2. Different organizational guidelines for reprocessing endoscopes were found to be similar. However, there were subtle differences, as shown in table 2. Reprocessing endoscopes shall be conducted in accordance to recommendations for each step of the reprocessing procedure, during both manual and automated techniques.
Table 2. Comparison of the reprocessing steps between guidelines
Questionnaire Results Analysis
The electronic questionnaire was developed and disseminated by the risk analysis section to healthcare facilities in Saudi Arabia. The questionnaire consisted of 12 questions aimed to assess the reprocessing of endoscopes locally. It covered critical reprocessing procedures and related incidents. The purpose of surveying healthcare facilities in Saudi Arabia was to evaluate the current practices and to form a set of recommendations to avoid endoscopes related outbreaks and to ensure the safety of both patients and healthcare workers.
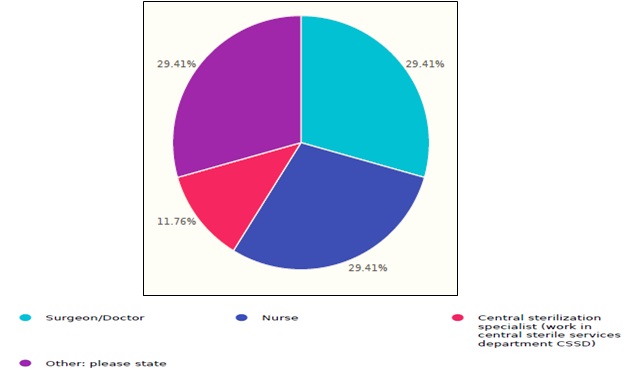
The total response number was 17 complete responses from healthcare facilities distributed over Saudi Arabia’s regions. The respondents had diversity of occupations such as; 29.4% surgeons/doctors, 29.4% nurses, 11.7% central sterilization specialists, and others, as shown in figure 1.
Other: please state
Neurosurgery, Endoscopy Clinical Specialist, Infection Prevention and Control Director, Two Biomedical Engineers
The most common endoscope used in Saudi healthcare facilities is colonoscope with a percentage of 76.4%, followed by gastroscope and bronchoscope with a percentage of 70.5% for both, as shown in the figure 2.
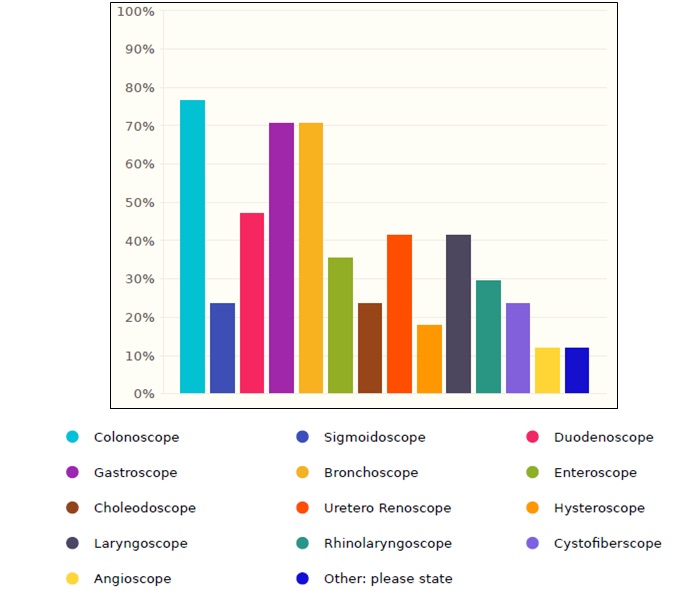
Figure 2. Flexible endoscopes distribution in Saudi healthcare facilities.
The results showed that 58.8% of the respondents follow certain guideline’s reprocessing procedures while the rest are not sure, as shown in figure 3. As per the given answers, HCFs follow several organizations’ guidelines which included: JSI, GCC, AAMI, SGNA guidelines.
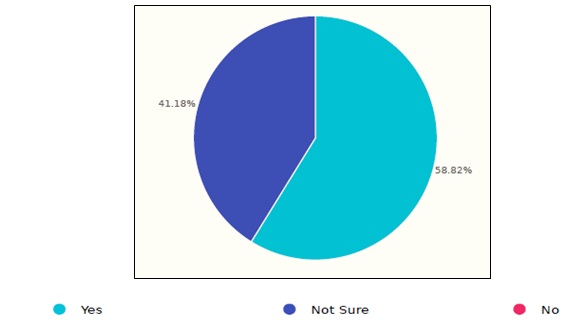
Figure 3. Followed guidelines in Saudi healthcare facilities.
Please provide the name for the regulation/guideline/ protocol:
- We follow manufacturer reprocessing guideline of scope and repressor device
- Guidelines of reprocessing medical instruments
- Upper GIT
- Clinical Internal Policy and / OR Procedure (CIPP) for cleaning & disinfecting endoscopic equipment
- JSI; JSI Research & Training Institute
- GCC Infection Prevention and Control Manual, 2nd Edition, 2013, Section VIII, ICM-VIII-04, Endoscopy.
- Infection Control and Epidemiology, Inc. (APIC) Chapter47
- AAMI
- SGNA
- Manufacturer IFU, hospital infection control guidelines
- Flexible endoscopes reprocessing
Documenting the sterilization procedures is one of the common steps in the aforementioned international guidelines. The results showed that 11.76% of the surveyed healthcare facilities do not document or track the reprocessing steps, as shown in the figure 4.
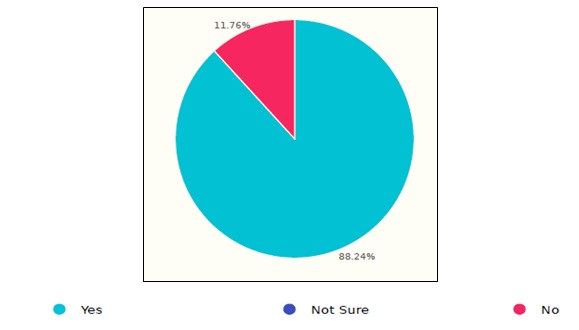
Figure 4. Documentation status of endoscope sterilization process in Saudi healthcare facilities.
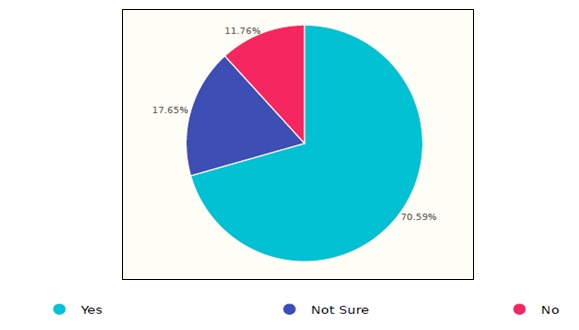
Figure 5. Saudi healthcare response to the storage procedure.
Incidence Cases of Endoscopes
Some of the survey questions were designed to discover issues that resulted in infection transmission within the healthcare facility caused by or related to the use of endoscopes. Figure 6 demonstrates the responses of 17 HCFs, in which the results showed two injuries/adverse events. Further questions clarified that the incidents happened at the time of sterilization. Nevertheless, an investigation should be performed to figure out whether or not the incidents, whenever confirmed, have been reported to the manufacturer and/or SFDA, following with that the SFDA requirements of reporting serious events that relate to medical devices.
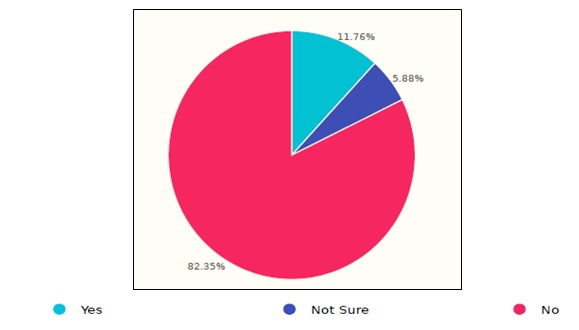
Figure 6. Incidence of endoscopes in Saudi healthcare facilities.
Part III: Conclusion
The previous results and analyses provide indications of the actual practices of reprocessing flexible endoscopes within the Saudi healthcare facilities. Such indications highlight primarily the following aspects:
- Saudi healthcare facilities follow different endoscopes reprocessing guidelines.
- Some healthcare facilities do not have a tracking system to document the processing procedure steps. Having a tracking system could be an essential element in preventing the transmission of infections.
After analyzing the responses of the device users in the Saudi market, the following set of actions are recommended:
- Add a requirement in the safe use of medical devices evaluation form to ensure compliance with the endoscope reprocessing procedures as indicated by the IFU and the manufacturer's recommendations.
Forming work team to create guidelines and standards that include best practices for Saudi healthcare providers to follow and reflect in procedures.
Thanks to the post-market clinical evaluation team for their supports in conducting this work. For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] Julia Kovaleva, Frans T M Peters, Henny C van der Mei, John E Degener, "Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy," NCBI, 2013.
[2] Nikki Kenters, Elisabeth G W Huijskens, Corianne Meier, Andreas Voss, "Infectious diseases linked to cross-contamination of flexible endoscopes," NCBI, 2015.
[3] N. Kenters, E. Tartari, J. Hopman, Rehab H. El-Sokkary, M. Nagao, K. Marimuthu, M. C. Vos, ISAC working group, E. G. W. Huijskens, Andreas Voss, "Worldwide Practices on Flexible Endoscope," Antimicrobial Resistance and Infection Control, 2018.
[4] Centers for Disease Control and Prevention, "Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the Healthcare Infection Control Practices Advisory Committee," 2017.
[5] World Gastroenterology Organisation, "Endoscope disinfection update: a guide to resource-sensitive reprocessing," 2019.
[6] The British Society of Gastroenterology, "Guidance on Decontamination of Equipment for Gastrointestinal Endoscopy".
[7] European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroentrology and Endoscopy Nurses and Associates (ESGENA), "Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA)," 2018.


