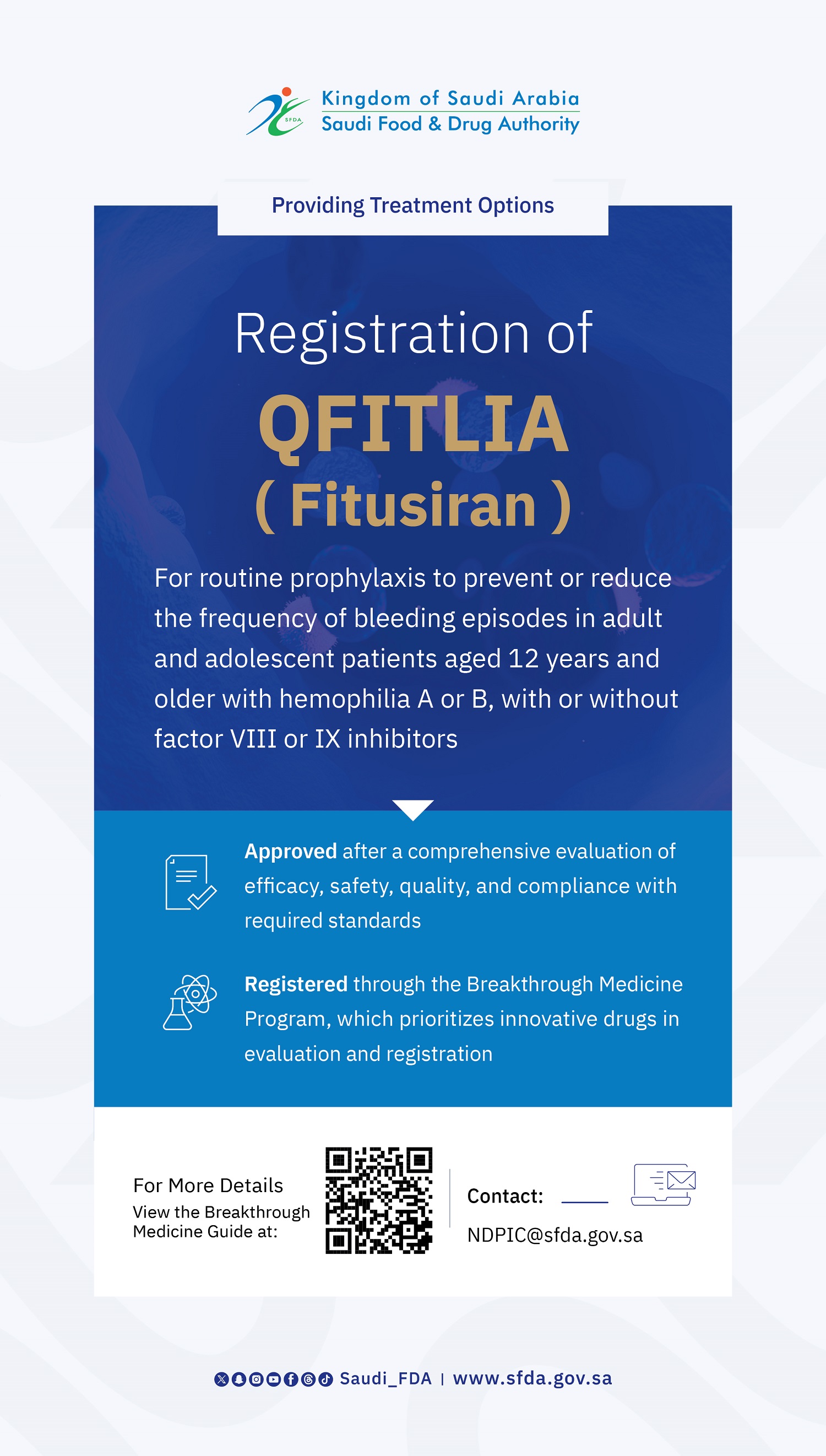
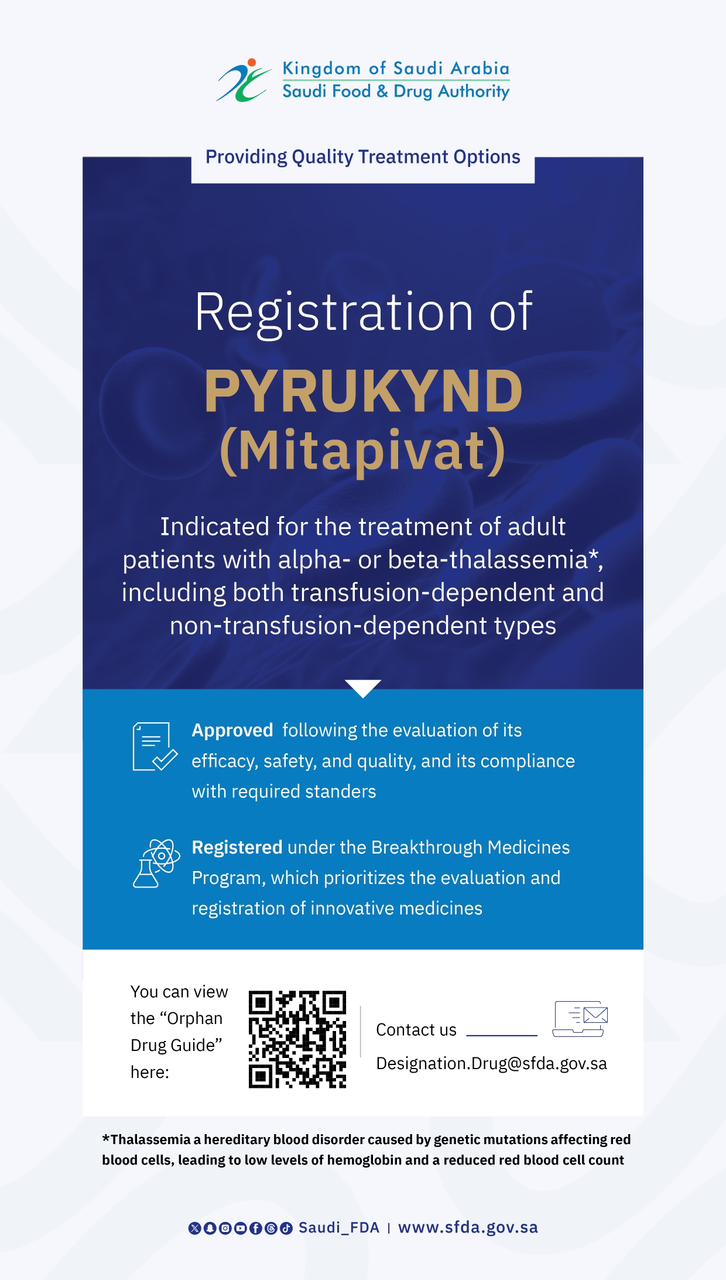
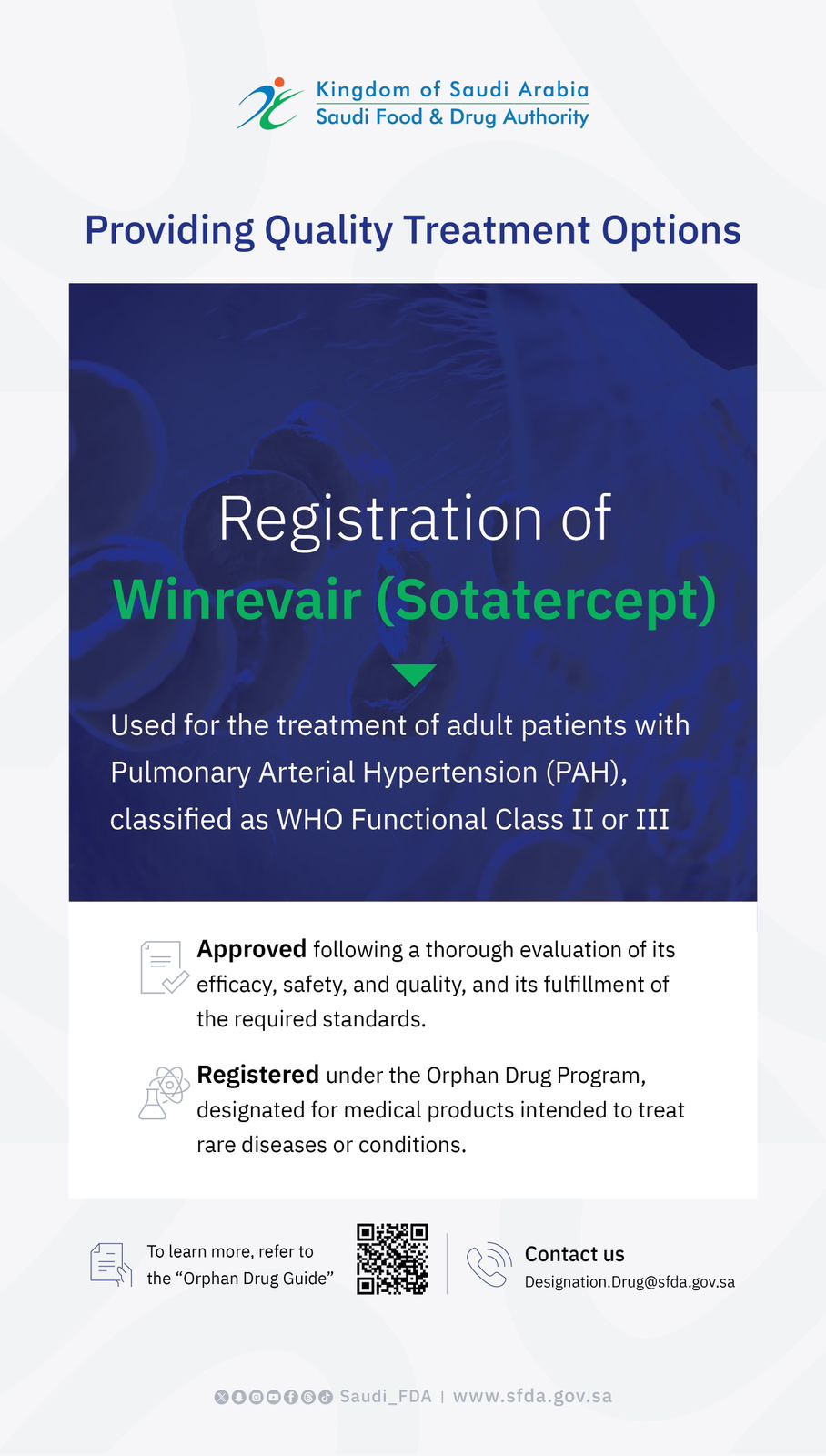
Saudi Innovation in Surgery: SFDA Grants Marketing Authorization for a Saudi-Made Medical Device
2025-08-17
The Saudi Food and Drug Authority (SFDA) has approved a groundbreaking, Saudi-developed medical device for marketing. This innovative tool is designed to improve the safety and efficiency of surgical procedures.
The device is a hand-worn tool that features a magnetic holder on the back of the hand to secure suture needles during knot-tying. This design allows surgeons to use both hands with ease, significantly reducing the risk of accidental needle injuries during operations. Before granting approval, the device underwent a thorough evaluation process, including a review of technical documentation and clinical evidence to ensure its safety and effectiveness.
Patents and Local Production
The SFDA explained that the device is protected by two registered patents in both the Kingdom of Saudi Arabia and the United States. Developed at King Saud University, it is being manufactured locally by a Saudi medical device factory, showcasing the Kingdom of Saudi Arabia's growing capacity for national innovation.
A Strategic Local Partnership Driving Medical Innovation
The project is a direct outcome of a strategic partnership between the SFDA and King Saud University’s Entrepreneurship Institute. The SFDA provided critical support through workshops and regulatory consultations to help the developers meet technical requirements. The private sector also played a key role in bringing the product to the Saudi market. This joint effort resulted in the product's successful market entry, highlighting the integrated roles of different sectors in fostering national creativity.
Paving the Way for Future Innovators
This achievement is one of the positive outcomes of the "Innovative Medical Devices" pathway, a program launched by the SFDA in 2021. The program aims to support and encourage innovators to continue developing medical technologies that strengthen the healthcare system and improve patient outcomes, bringing new solutions to life.
Enhancing Saudi Leadership in Medical Innovation
The approval of this device marks a significant step forward for the healthcare sector in Saudi Arabia. It directly supports the Healthcare Transformation Program, a key part of Saudi Vision 2030, which seeks to build a sustainable healthcare system and create a regulatory environment that inspires innovation. This success reinforces the Kingdom's position as a leader in global medical research and development.